Diagnostic de la maladie cœliaque

Le diagnostic définitif de la maladie cœliaque s’appuie sur quatre éléments fondamentaux. Il est important que le patient ait une alimentation normale, avant d’effectuer le diagnostic.
Le diagnostic de la maladie cœliaque passe par quatre étapes. En tout premier lieu, une anamnèse répertoriant la présence des troubles, suivie d’analyses sérologiques et histologiques. Et, en tout dernier lieu, une période d’alimentation sans gluten qui viendra confirmer le diagnostic.
I. Anamnèse
L'anamnèse s’intéresse aux antécédents familiaux, au mode d’alimentation, en particulier, aux aliments contenant du gluten. Une liste des troubles cliniques apparus est dressée, pointant les symptômes que sont diarrhées, perte de poids, fatigue, ballonnement abdominal, maux de ventre, nausées et retards de croissance chez les enfants
II. Sérologie
Le Gold standard des analyses sérologiques réside dans la recherche des marqueurs de la maladie : les anticorps anti-transglutaminases IgA (tTg-IgA). En cas de résultats négatifs, on complète les analyses par une recherche des anticorps anti-transglutaminases de classe IgG (tTg-IgG), complémentée par une recherche des anticorps anti-endomysium de classe IgA (EMA). Habituellement, on effectue toujours un dosage des IgA totaux, car un fort pourcentage (3 à 11 %) des personnes souffrant de la maladie cœliaque présentent un déficit en IgA. En cas de déficit en IgA, le diagnostic sérologique des anticorps IgA ne permet pas de tirer de conclusions.
III. Histologie
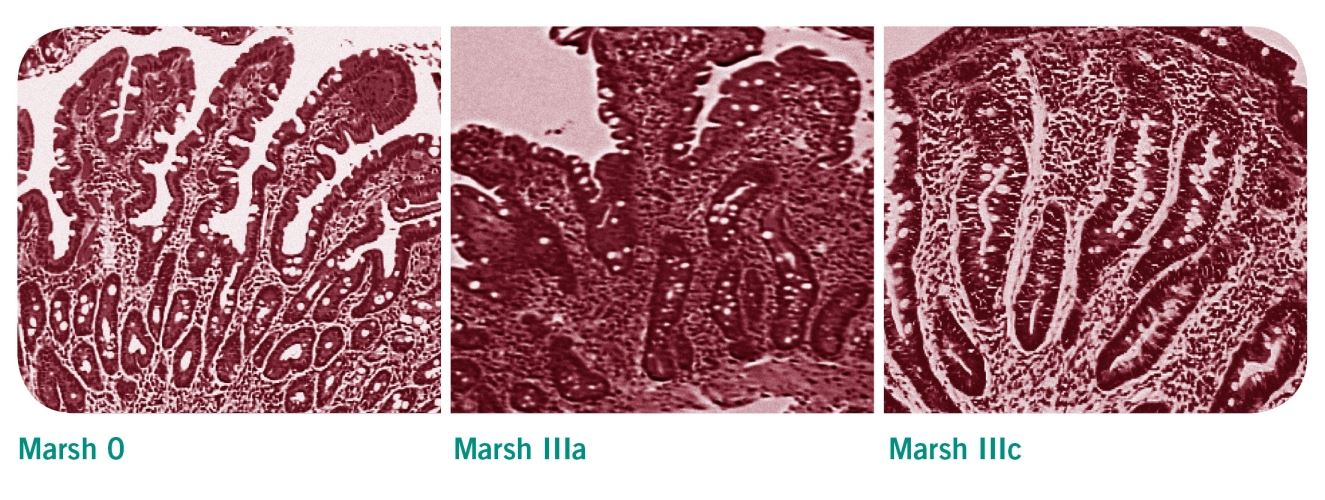
L'analyse histologique comprend tout d’abord une œsophago-gastro-duodénoscopie (OGD, c'est-à-dire un examen endoscopique de l'œsophage, de l'estomac et de l'intestin) ainsi qu’une biopsie au biveau du bulbe ou du 2ème duodénum. On prélèvera au moins trois échantillons de la partie descendante du duodénum, ainsi qu’un autre à l’angle entre le duodénum et le jéjunum. Les modifications histologiques de ces échantillons sont répertoriées d’après la classification de Marsh. En cas de maladie cœliaque, on observe un nombre accru des lymphocytes intra-épithéliaux (LIE) ainsi qu'une hypertrophie/hyperplasie et un allongement des cryptes. Les villosités de l’intestin grêle des personnes souffrant de la maladie cœliaque sont plus courtes, moins nombreuses voire inexistantes. Cela correspond à la classification de Marsh IIIa et IIIc.
Classification de Marsh
- Marsh 0: Muqueuses et cellules épithéliales intactes de l’intestin grêle
- Marsh I: Augmentation des lymphocytes intra-épithéliaux (LIE) >30% cellules épithéliales
- Marsh II: Augmentation des LIE et hyperplasie des cryptes
- Marsh IIIa à IIIc: Augmentation des LIE et hyperplasie des cryptes, atrophie villaire partielle dans la classe III A, sub-totale dans la classe III B et totale dans la classe III C.
Biopsie chez les enfants ?
Une biopsie de l'intestin grêle est nécessaire afin d’établir un diagnostic fiable sauf chez les enfants qui, d’après les directives de l’ESPGHAN, présentent les symptômes et les signes cliniques typiques d'une malabsorption. En outre, d‘autres facteurs déclencheurs doivent avoir été mis en évidence telle que l’augmentation du taux d’anticorps anti-transglutaminases (dix fois supérieure à la moyenne), ce qui permettra, en accord avec le gastroentérologue, de se passer d’une biopsie.
IV. Période d’observation pendant le régime sans gluten
Une fois les examens sérologiques et histologiques effectués, les aliments contenant du gluten sont totalement banni de l’alimentation quotidienne. Le diagnostic n’est confirmé qu’en cas d’amélioration des symptômes et si une nouvelle sérologie démontre que les anticorps réagissent à l'alimentation sans gluten. Pour les personnes diagnostiqués récemment, il est essentiel de s’assurer du soutien d’un nutritionniste pour la mise en place du régime.
Contrôles et suivis réguliers
On recommande aux personnes souffrant de la maladie cœliaque d’effectuer un bilan de contrôle une fois par an afin de prévenir les complications et les carences. À cette occasion, il faut effectuer un dosage des tTg-IgA afin de s’assurer que le régime sans gluten est bien observé. Un résultat négatif concernant les anticorps indique une bonne observance. Lorsque le patient réagit positivement à l'alimentation sans gluten, une nouvelle biopsie de l'intestin grêle n'est qu'exceptionnellement préconisée.
Suivi et contrôles réguliers recommandés:
Suivi et contrôles réguliers recommandés:
- Trois mois après avoir établi le diagnostic
- Un an plus tard
- Puis tous les ans
Plus d'informations à ce sujet
Téléchargement
2
Montrer tout
Études
6
Montrer tout

Fichier « Conseils et outils pour la prise en charge diététique de la maladie coeliaque »

Maladie coeliaque - Une maladie sous diagnostiquée

A Retrospective Application of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosis of Coeliac Disease in New Zealand Children.
In 2012 the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) published an updated guideline for the diagnosis of Coeliac disease, proposing a non-biopsy based diagnostic approach for a proportion of symptomatic children where appropriate serology (tTG IgA level at least 10 times upper limit of normal and positive EMA) and 'at risk' genotype (HLA DQ2 or DQ8) was evident. This retrospective study aimed to assess the applicability of this guideline to children in New Zealand. Children less than 16 years of age investigated for coeliac disease with small bowel biopsy in between January 2010 and December 2012 were identified. The results of those with tissue transglutaminase IgA (tTG) levels greater than 50 units (10 times upper limit of normal), positive endomysial antibodies and HLA DQ2/DQ8 were used to calculate sensitivity and specificity. Data from 160 children was available: 70 had biopsy-confirmed Coeliac disease, and 90 had negative biopsies. Limited data relating to HLA DQ2/DQ8 testing precluded application of the guidelines to all patients. However, the isolated use of the anti-tTG antibody cut off of 10 times the upper limit of normal generated a sensitivity of 67% and moderately high specificity of 92%. Specificity increased to 97% when limited EMA and HLA DQ2/DQ8 data was added. data, levels above 50 units and a specificity of 92%. Specificity increased to 97% when limited EMA and HLA DQ2/DQ8 data was added. A potential 67% reduction in the number of diagnostic biopsies performed would significantly reduce the work load and cost to the health system along with inconvenience and stress to families. However, it is important to avoid incorrect diagnosis of coeliac disease - if this current study is representative of the local population, up to three children out of every hundred investigated for coeliac disease could be falsely diagnosed. The limited amount of data was a major limitation of this study, therefore the value of the 2012 ESPGHAN guideline for the diagnosis of coeliac disease should now be evaluated prospectively.
Resource: International Journal of Coeliac Disease, Dec 2014 (vol 2; no. 4)
Resource: International Journal of Coeliac Disease, Dec 2014 (vol 2; no. 4)

Coeliac disease: The debate on coeliac disease screening - are we there yet?
The majority of patients with coeliac disease are undiagnosed, leading to debate about the utility of screening. The heterogeneous clinical presentation, which includes asymptomatic forms, can partially explain the difficulties faced when identifying coeliac disease. Now, Kurppa and colleagues add another element to the debate by strengthening the arguments for general screening.
Resource: Nature Reviews Gastroenterology & Hepatology Volume: 11, Pages: 457–458 201
Resource: Nature Reviews Gastroenterology & Hepatology Volume: 11, Pages: 457–458 201

Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology
ABSTRACT
A multidisciplinary panel of 18 physicians and 3 non-physicians from eight countries (Sweden, UK, Argentina, Australia, Italy, Finland, Norway and the USA) reviewed the literature on diagnosis and management of adult coeliac disease (CD).
This paper presents the recommendations of the British Society of Gastroenterology. Areas of controversies were explored through phone meetings and web surveys. Nine working groups examined the following areas of CD diagnosis and management: classification of CD; genetics and immunology; diagnostics; serology and endoscopy; follow-up; gluten-free diet; refractory CD and malignancies; quality of life; novel treatments; patient support; and screening for CD.
Resource: Gut doi:10.1136/gutjnl-2013-306578
Jonas F Ludvigsson, Julio C Bai, Federico Biagi, Timothy R Card, Carolina Ciacci, Paul J Ciclitira, Peter H R Green, Marios Hadjivassiliou, Anne Holdoway, David A van Heel, Katri Kaukinen, Daniel A Leffler, Jonathan N Leonard, Knut E A Lundin, Norma McGough, Mike Davidson, Joseph A Murray, Gillian L Swift, Marjorie M Walker, Fabiana Zingone, David S Sanders, Authors of the BSG Coeliac Disease Guidelines Development Group
A multidisciplinary panel of 18 physicians and 3 non-physicians from eight countries (Sweden, UK, Argentina, Australia, Italy, Finland, Norway and the USA) reviewed the literature on diagnosis and management of adult coeliac disease (CD).
This paper presents the recommendations of the British Society of Gastroenterology. Areas of controversies were explored through phone meetings and web surveys. Nine working groups examined the following areas of CD diagnosis and management: classification of CD; genetics and immunology; diagnostics; serology and endoscopy; follow-up; gluten-free diet; refractory CD and malignancies; quality of life; novel treatments; patient support; and screening for CD.
Resource: Gut doi:10.1136/gutjnl-2013-306578
Jonas F Ludvigsson, Julio C Bai, Federico Biagi, Timothy R Card, Carolina Ciacci, Paul J Ciclitira, Peter H R Green, Marios Hadjivassiliou, Anne Holdoway, David A van Heel, Katri Kaukinen, Daniel A Leffler, Jonathan N Leonard, Knut E A Lundin, Norma McGough, Mike Davidson, Joseph A Murray, Gillian L Swift, Marjorie M Walker, Fabiana Zingone, David S Sanders, Authors of the BSG Coeliac Disease Guidelines Development Group

Serological Assessment for Celiac Disease in IgA Deficient Adults
Abstract
Purpose: Selective immunoglobulin A deficiency is the most common primary immunodeficiency disorder that is strongly overrepresented among patients with celiac disease (CD). IgG antibodies against tissue transglutaminase (tTG) and deamidated gliadin peptides (DGP) serve as serological markers for CD in IgA deficient individuals, although the diagnostic value remains uncertain. The aim of this study was to investigate the prevalence of these markers in a large cohort of IgA deficient adults with confirmed or suspected CD and relate the findings to gluten free diet.
Methods: Sera from 488,156 individuals were screened for CD in seven Swedish clinical immunology laboratories between 1998 and 2012. In total, 356 out of 1,414 identified IgA deficient adults agreed to participate in this study and were resampled. Forty-seven IgA deficient blood donors served as controls. Analyses of IgG antibodies against tTG and DGP as well as HLA typing were performed and a questionnaire was used to investigate adherence to gluten free diet. Available biopsy results were collected.
Results: Out of the 356 IgA deficient resampled adults, 67 (18.8%) were positive for IgG anti-tTG and 79 (22.2%) for IgG anti-DGP, 54 had biopsy confirmed CD. Among the 47 IgA deficient blood donors, 4 (9%) were positive for IgG anti-tTG and 8 (17%) for anti-DGP. Four were diagnosed with biopsy verified CD, however, 2 of the patients were negative for all markers. Sixty-eight of 69 individuals with positive IgG anti-tTG were HLA-DQ2/DQ8 positive whereas 7 (18.9%) of the 37 individuals positive for IgG anti-DGP alone were not.
Conclusions: IgG anti-tTG seems to be a more reliable marker for CD in IgA deficient adults whereas the diagnostic specificity of anti-DGP appears to be lower. High levels of IgG antibodies against tTG and DGP were frequently found in IgA deficient adults despite adhering to gluten free diet.
Resource: PLOS ONE, 9 April 2014, Vol 9, Issue 4 (www.plosone.org)
Purpose: Selective immunoglobulin A deficiency is the most common primary immunodeficiency disorder that is strongly overrepresented among patients with celiac disease (CD). IgG antibodies against tissue transglutaminase (tTG) and deamidated gliadin peptides (DGP) serve as serological markers for CD in IgA deficient individuals, although the diagnostic value remains uncertain. The aim of this study was to investigate the prevalence of these markers in a large cohort of IgA deficient adults with confirmed or suspected CD and relate the findings to gluten free diet.
Methods: Sera from 488,156 individuals were screened for CD in seven Swedish clinical immunology laboratories between 1998 and 2012. In total, 356 out of 1,414 identified IgA deficient adults agreed to participate in this study and were resampled. Forty-seven IgA deficient blood donors served as controls. Analyses of IgG antibodies against tTG and DGP as well as HLA typing were performed and a questionnaire was used to investigate adherence to gluten free diet. Available biopsy results were collected.
Results: Out of the 356 IgA deficient resampled adults, 67 (18.8%) were positive for IgG anti-tTG and 79 (22.2%) for IgG anti-DGP, 54 had biopsy confirmed CD. Among the 47 IgA deficient blood donors, 4 (9%) were positive for IgG anti-tTG and 8 (17%) for anti-DGP. Four were diagnosed with biopsy verified CD, however, 2 of the patients were negative for all markers. Sixty-eight of 69 individuals with positive IgG anti-tTG were HLA-DQ2/DQ8 positive whereas 7 (18.9%) of the 37 individuals positive for IgG anti-DGP alone were not.
Conclusions: IgG anti-tTG seems to be a more reliable marker for CD in IgA deficient adults whereas the diagnostic specificity of anti-DGP appears to be lower. High levels of IgG antibodies against tTG and DGP were frequently found in IgA deficient adults despite adhering to gluten free diet.
Resource: PLOS ONE, 9 April 2014, Vol 9, Issue 4 (www.plosone.org)

Coeliac Patients Are Undiagnosed at Routine Upper Endoscopy
Abstract
Background and Aims
Two out of three patients with Coeliac Disease (CD) in Australia are undiagnosed. This prospective clinical audit aimed to determine how many CD patients would be undiagnosed if duodenal biopsy had only been performed if the mucosa looked abnormal or the patient presented with typical CD symptoms.
Methods
All eligible patients presenting for upper gastrointestinal endoscopy (OGD) in a regional center from 2004–2009 underwent prospective analysis of presenting symptoms and duodenal biopsy. Clinical presentations were defined as either Major (diarrhea, weight loss, iron deficiency, CD family history or positive celiac antibodies- Ab) or Minor Clinical Indicators (CI) to duodenal biopsy (atypical symptoms). Newly diagnosed CD patients had follow up celiac antibody testing.
Results
Thirty-five (1.4%) new cases of CD were identified in the 2,559 patients biopsied at upper endoscopy. Almost a quarter (23%) of cases presented with atypical symptoms. There was an inverse relationship between presentation with Major CI’s and increasing age (<16, 16–59 and >60: 100%, 81% and 50% respectively, p = 0.03); 28% of newly diagnosed CD patients were aged over 60 years. Endoscopic appearance was a useful diagnostic tool in only 51% (18/35) of CD patients. Coeliac antibodies were positive in 34/35 CD patients (sensitivity 97%).
Conclusions
Almost one quarter of new cases of CD presented with atypical symptoms and half of the new cases had unremarkable duodenal mucosa. At least 10% of new cases of celiac disease are likely to be undiagnosed at routine upper endoscopy, particularly patients over 60 years who more commonly present atypically. All new CD patients could be identified in this study by performing pre-operative celiac antibody testing on all patients presenting for OGD and proceeding to biopsy only positive antibody patients and those presenting with either Major CI or abnormal duodenal mucosa for an estimated cost of AUS$4,629 and AUS$3,710 respectively.
Resource: PLOS ONE, March 2014, Vol 9, Issue 3 (www.plosone.org)
Background and Aims
Two out of three patients with Coeliac Disease (CD) in Australia are undiagnosed. This prospective clinical audit aimed to determine how many CD patients would be undiagnosed if duodenal biopsy had only been performed if the mucosa looked abnormal or the patient presented with typical CD symptoms.
Methods
All eligible patients presenting for upper gastrointestinal endoscopy (OGD) in a regional center from 2004–2009 underwent prospective analysis of presenting symptoms and duodenal biopsy. Clinical presentations were defined as either Major (diarrhea, weight loss, iron deficiency, CD family history or positive celiac antibodies- Ab) or Minor Clinical Indicators (CI) to duodenal biopsy (atypical symptoms). Newly diagnosed CD patients had follow up celiac antibody testing.
Results
Thirty-five (1.4%) new cases of CD were identified in the 2,559 patients biopsied at upper endoscopy. Almost a quarter (23%) of cases presented with atypical symptoms. There was an inverse relationship between presentation with Major CI’s and increasing age (<16, 16–59 and >60: 100%, 81% and 50% respectively, p = 0.03); 28% of newly diagnosed CD patients were aged over 60 years. Endoscopic appearance was a useful diagnostic tool in only 51% (18/35) of CD patients. Coeliac antibodies were positive in 34/35 CD patients (sensitivity 97%).
Conclusions
Almost one quarter of new cases of CD presented with atypical symptoms and half of the new cases had unremarkable duodenal mucosa. At least 10% of new cases of celiac disease are likely to be undiagnosed at routine upper endoscopy, particularly patients over 60 years who more commonly present atypically. All new CD patients could be identified in this study by performing pre-operative celiac antibody testing on all patients presenting for OGD and proceeding to biopsy only positive antibody patients and those presenting with either Major CI or abnormal duodenal mucosa for an estimated cost of AUS$4,629 and AUS$3,710 respectively.
Resource: PLOS ONE, March 2014, Vol 9, Issue 3 (www.plosone.org)

Celiac disease: diagnosis and management.
Abstract
Celiac disease is an autoimmune disorder of the gastrointestinal tract. It is triggered by exposure to dietary gluten in genetically susceptible individuals. Gluten is a storage protein in wheat, rye, and barley, which are staples in many American diets. Celiac disease is characterized by chronic inflammation of the small intestinal mucosa, which leads to atrophy of the small intestinal villi and subsequent malabsorption. The condition may develop at any age. Intestinal manifestations include diarrhea and weight loss. Common extraintestinal manifestations include iron deficiency anemia, decreased bone mineral density, and neuropathy. Most cases of celiac disease are diagnosed in persons with extraintestinal manifestations. The presence of dermatitis herpetiformis is pathognomonic for celiac disease. Diagnosis is supported by a positive tissue transglutaminase serologic test but, in general, should be confirmed by a small bowel biopsy showing the characteristic histology associated with celiac disease. The presence of human leukocyte antigen alleles DQ2, DQ8, or both is essential for the development of celiac disease, and can be a useful genetic test in select instances. Treatment of celiac disease is a gluten-free diet. Dietary education should focus on identifying hidden sources of gluten, planning balanced meals, reading labels, food shopping, dining out, and dining during travel. About 5% of patients with celiac disease are refractory to a gluten-free diet. These patients should be referred to a gastroenterologist for reconsideration of the diagnosis or for aggressive treatment of refractory celiac disease, which may involve corticosteroids and immunomodulators.
Resource: Am Fam Physician. 2014 Jan 15;89(2):99-105.
Celiac disease is an autoimmune disorder of the gastrointestinal tract. It is triggered by exposure to dietary gluten in genetically susceptible individuals. Gluten is a storage protein in wheat, rye, and barley, which are staples in many American diets. Celiac disease is characterized by chronic inflammation of the small intestinal mucosa, which leads to atrophy of the small intestinal villi and subsequent malabsorption. The condition may develop at any age. Intestinal manifestations include diarrhea and weight loss. Common extraintestinal manifestations include iron deficiency anemia, decreased bone mineral density, and neuropathy. Most cases of celiac disease are diagnosed in persons with extraintestinal manifestations. The presence of dermatitis herpetiformis is pathognomonic for celiac disease. Diagnosis is supported by a positive tissue transglutaminase serologic test but, in general, should be confirmed by a small bowel biopsy showing the characteristic histology associated with celiac disease. The presence of human leukocyte antigen alleles DQ2, DQ8, or both is essential for the development of celiac disease, and can be a useful genetic test in select instances. Treatment of celiac disease is a gluten-free diet. Dietary education should focus on identifying hidden sources of gluten, planning balanced meals, reading labels, food shopping, dining out, and dining during travel. About 5% of patients with celiac disease are refractory to a gluten-free diet. These patients should be referred to a gastroenterologist for reconsideration of the diagnosis or for aggressive treatment of refractory celiac disease, which may involve corticosteroids and immunomodulators.
Resource: Am Fam Physician. 2014 Jan 15;89(2):99-105.

A Retrospective Application of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosis of Coeliac Disease in New Zealand Children.
In 2012 the European Society for Pediatric Gastroenterology, Hepatolog...

Coeliac disease: The debate on coeliac disease screening - are we there yet?
The majority of patients with coeliac disease are undiagnosed, leading...

Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology
ABSTRACT
A multidisciplinary panel of 18 physicians and 3 non-physi...

Serological Assessment for Celiac Disease in IgA Deficient Adults
Abstract
Purpose: Selective immunoglobulin A deficiency is the most...

Coeliac Patients Are Undiagnosed at Routine Upper Endoscopy
Abstract
Background and Aims
Two out of three patients with Coelia...

Celiac disease: diagnosis and management.
Abstract
Celiac disease is an autoimmune disorder of the gastrointe...
www.drschaer-institute.com
