Coexisting conditions of coeliac disease

Evidence suggests that people with certain other medical conditions may have an increased risk of developing coeliac disease during their lifetime. The relationship between coeliac disease and it’s co-existing conditions offers important opportunities for screening amongst certain groups.
A list of the conditions associated with coeliac disease is provided below. There is good evidence that coeliac disease has an increased prevalence amongst patients with autoimmune thyroid disease (up to 7%), irritable bowel syndrome (up to 7%), and type 1 diabetes (2-10%). For this reason, it is recommended that adults and children with these conditions are offered serological testing for coeliac disease [1].
Furthermore, there is ‘some’ evidence that coeliac disease has an increased prevalence in people with [1]:
- Addison’s Disease
- Autoimmune liver conditions
- Autoimmune myocarditis
- Chronic thrombocytopenia purpura
- Depression/bipolar disorder
- Downs Syndrome
- Epilepsy
- Lymphoid malignancy
- Polyneuropathy
- Sjögren’s Syndrome
- Sarcoidosis
- Turner Syndrome
- Unexplained subfertility
Type 1 diabetes
Coeliac disease and type 1 diabetes often occur together. The prevalence of coeliac disease in persons with Type 1 diabeties is between 2% and 10%, depending on the age of the patient [1]. Coeliac disease also regularly occurs in first-degree relatives of type 1 diabetics.
Genetic similarities
The joint occurrence of the two diseases seems to be caused by a common genetic predisposition, namely, the occurrence of HLA-DQ2 and HLA-DQ8, known as histocompatibility genes.
Screening for coeliac disease amongst type 1 diabetics
Similarly to coeliac disease, the incidence of type 1 diabetes is rising, with a reported increase of 3% annually [2]. Less than 10% of patients with type 1 diabetes and coeliac disease report gastrointestinal symptoms [3], hence the recommendation to routinely screen for coeliac disease amongst this patient group. There is conflicting recommendations on how often repeat screening should be conducted. Current ESPGHAN guidelines recommend offering repeat screening every 2-3 years [4], whereas NICE suggest that the evidence is insufficient to recommend specific intervals for repeat screening following initial testing at diabetes diagnosis. In the vast majority of patients, coeliac diagnosis follows the diagnosis of type 1 diabetes.
Impact of a dual diagnosis
Evidence suggests that children with both type 1 diabetes and coeliac disease have lower weight and height standard deviation scores 5. Furthermore, good adherence to a gluten free diet appears to result in steady improvement in height and weight whilst poor adherence is likely to result in continued growth impairment [6]. Among adults newly diagnosed with coeliac disease, glycaemic control has been shown to be significantly worse than amongst those with type 1 diabetes alone [7], although this effect may be reduced over time as the gluten free diet becomes well established [5,8]. Malabsorption of carbohydrates secondary to mucosal damage may explain the observation that episodes of hypoglycaemia are more common amongst patients with type 1 diabetes and coeliac disease in the six months before and after coeliac diagnosis. Once again, this finding is not repeated upon longer term follow up of patients, indicating gut recovery on a gluten free diet [9].
Long term risk
Unfortunately the prevalence of longer term complications associated with diabetes appear to be significantly greater for patients with both conditions, compared to those with diabetes/coeliac disease alone. Both risk of retinopathy [10] and subclinical atherosclerosis [11] is increased for those with a dual diagnosis in the longer term. Overall mortality risk after the first 5 years post coeliac diagnosis also appears to increase over time for those with both type 1 diabetes and coeliac disease [12].
Dietary therapy for diabetes and coeliac disease
A strict gluten free diet is required in order to maintain glycaemic control and reduce the short and long term complications of both diseases. The dietary management of both conditions is based on general healthy eating principles that apply to the population as a whole for the purpose of disease risk reduction and promotion of optimal health. Weight control and exercise are also paramount to glycaemic control.
Genetic similarities
The joint occurrence of the two diseases seems to be caused by a common genetic predisposition, namely, the occurrence of HLA-DQ2 and HLA-DQ8, known as histocompatibility genes.
Screening for coeliac disease amongst type 1 diabetics
Similarly to coeliac disease, the incidence of type 1 diabetes is rising, with a reported increase of 3% annually [2]. Less than 10% of patients with type 1 diabetes and coeliac disease report gastrointestinal symptoms [3], hence the recommendation to routinely screen for coeliac disease amongst this patient group. There is conflicting recommendations on how often repeat screening should be conducted. Current ESPGHAN guidelines recommend offering repeat screening every 2-3 years [4], whereas NICE suggest that the evidence is insufficient to recommend specific intervals for repeat screening following initial testing at diabetes diagnosis. In the vast majority of patients, coeliac diagnosis follows the diagnosis of type 1 diabetes.
Impact of a dual diagnosis
Evidence suggests that children with both type 1 diabetes and coeliac disease have lower weight and height standard deviation scores 5. Furthermore, good adherence to a gluten free diet appears to result in steady improvement in height and weight whilst poor adherence is likely to result in continued growth impairment [6]. Among adults newly diagnosed with coeliac disease, glycaemic control has been shown to be significantly worse than amongst those with type 1 diabetes alone [7], although this effect may be reduced over time as the gluten free diet becomes well established [5,8]. Malabsorption of carbohydrates secondary to mucosal damage may explain the observation that episodes of hypoglycaemia are more common amongst patients with type 1 diabetes and coeliac disease in the six months before and after coeliac diagnosis. Once again, this finding is not repeated upon longer term follow up of patients, indicating gut recovery on a gluten free diet [9].
Long term risk
Unfortunately the prevalence of longer term complications associated with diabetes appear to be significantly greater for patients with both conditions, compared to those with diabetes/coeliac disease alone. Both risk of retinopathy [10] and subclinical atherosclerosis [11] is increased for those with a dual diagnosis in the longer term. Overall mortality risk after the first 5 years post coeliac diagnosis also appears to increase over time for those with both type 1 diabetes and coeliac disease [12].
Dietary therapy for diabetes and coeliac disease
A strict gluten free diet is required in order to maintain glycaemic control and reduce the short and long term complications of both diseases. The dietary management of both conditions is based on general healthy eating principles that apply to the population as a whole for the purpose of disease risk reduction and promotion of optimal health. Weight control and exercise are also paramount to glycaemic control.
Dermatitis herpetiformis
Dermatitis herpetiformis is the cutaneous manifestation of coeliac disease triggered by the ingestion of dietary gluten. It is characterised by the appearance of a patchy, itchy rash and small blisters, most commonly found on the elbows, knees, buttocks and scalp. Unlike coeliac disease, it is more common in men than in women with an overall population prevalence of 1:3,300. Less than 10% of patients with dermatitis herpetiformis have gut symptoms, but most have total or subtotal villous atrophy upon histological examination [13]. As in coeliac disease, virtually all patients with Dermatitis Herpetiformis carry the HLA DQ2/DQ8 alleles.
Treatment of Dermatitis Herpetiformis
A strict, life-long gluten-free diet is the mainstay of treatment for this condition. In the first month after the diagnosis or in the inflammatory phases of the disease, in which a gluten-free diet alone may not be enough to control the symptoms, several drugs can be used for variable periods of time, including Dapsone, sulfones or steroids. More than 70% of patients following a strict gluten free diet are able to slowly reduce their drug dose over a period of 2 years [14].
Treatment of Dermatitis Herpetiformis
A strict, life-long gluten-free diet is the mainstay of treatment for this condition. In the first month after the diagnosis or in the inflammatory phases of the disease, in which a gluten-free diet alone may not be enough to control the symptoms, several drugs can be used for variable periods of time, including Dapsone, sulfones or steroids. More than 70% of patients following a strict gluten free diet are able to slowly reduce their drug dose over a period of 2 years [14].
References
- NICE Guideline NG20: Recognition, Assessment & Management of Coeliac Disease. National Institute of Clinical Excellence 2015.
- Dabelea D, Mayer-Davis EJ, Saydah S et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014; 311:1778-1786
- Scaramuzza AE, Mantegazza C, Bosetti A, Zuccotti GV. Type 1 diabetes and celiac disease: The effects of gluten free diet on metabolic control. World J Diabetes 2013; 4: 130-134
- Husby S, Koletzko S, Korponay-Szabó IR et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54: 136-160
- Fröhlich-Reiterer EE, Kaspers S, Hofer S, et al. Anthropometry, metabolic control, and follow-up in children and adolescents with type 1 diabetes mellitus and biopsy-proven celiac disease. J Pediatr 2011; 158: 589-593.
- Akirov A, Hirsch G, Boyko V, et al. Celiac in type 1 diabetes children and youth–prevalence, metabolic control and growth parameters. Tel Aviv: Israel Society of Clinical Pediatrics, 2010
- Leeds JS, Hopper AD, Hadjivassiliou M, Tesfaye S, Sanders DS. High prevalence of microvascular complications in adults with type 1 diabetes and newly diagnosed celiac disease. Diabetes Care 2011; 34: 2158-2163
- Hansen D, Brock-Jacobsen B, Lund E, et al. Clinical benefit of a gluten-free diet in type 1 diabetic children with screening-detected celiac disease: a population-based screening study with 2 years’ follow-up. Diabetes Care 2006; 29: 2452-2456
- Mohn A, Cerruto M, Iafusco D, et al. Celiac disease in children and adolescents with type I diabetes: importance of hypoglycemia. J Pediatr Gastroenterol Nutr 2001; 32: 37-4
- Mollazadegan K, Kugelberg M, Montgomery SM, et al. population-based study of the risk of diabetic retinopathy in patients with type 1 diabetes and celiac disease. Diabetes Care 2013; 36: 316-321
- Pitocco D, Giubilato S, Martini F, et al. Combined atherogenic effects of celiac disease and type 1 diabetes mellitus. Atherosclerosis 2011; 217: 531-535
- Mollazadegan K, Sanders DS, Ludvigsson J, Ludvigsson JF. Long-term coeliac disease influences risk of death in patients with type 1 diabetes. J Intern Med 2013; 274: 273-280
- Fry L, Seah PP, Harper PG et al.The small intestine in dermatitis herpetiformis. J Clin Pathol 1974; 27:817-24
- Fry L. Leonard JN, Swain F et al. Long term follow up of dermatitis herpetiformis with and without dietary gluten withdrawal. Br J Dermatol 1982; 107:631-40.
Further information on this topic
Presentations
1
Show all
Studies
7
Show all

Gluten and Gut-Brain Axis: Lesson Learned From Autism And Schizophrenia (2015)
Alessio Fasano, M.D.
Mucosal Immunology and Biology Research Center
Center for Celiac Research
Massachusetts General Hospital for Children
16th International Coeliac Disease Symposium 2015 in Prague
Pre-Conference Workshop on Gluten Sensitivity "The Evolving Planet of Gluten Related Disorders"
Mucosal Immunology and Biology Research Center
Center for Celiac Research
Massachusetts General Hospital for Children
16th International Coeliac Disease Symposium 2015 in Prague
Pre-Conference Workshop on Gluten Sensitivity "The Evolving Planet of Gluten Related Disorders"

Gluten and Gut-Brain Axis: Lesson Learned From Autism And Schizophrenia (2015)
Alessio Fasano, M.D.
Mucosal Immunology and Biology Research Center
...
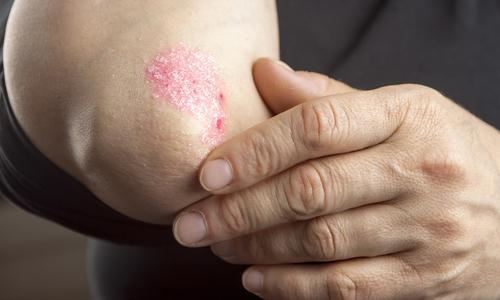
Association between Coeliac Disease and Psoriasis: Italian Primary Care Multicentre Study
Summary
Coeliac disease is increasingly associated with extraintestinal manifestations, among these, several skin disorders have been reported, with dermatitis herpetiformis being the best recognised. Several studies investigating the possible association between CD and psoriasis have been published, however results have so far proven to be inconclusive and most of the data on the effect of a gluten free diet on psoriatic skin lesions are contained within case reports.
In this prospective multicentre study, the patient records from 19 Italian primary care practices were screened for confirmed cases of psoriasis, following this 219 patients with a confirmed diagnosis of psoriasis were enrolled in to the study (111 males, 51%; mean age 54 years), and 264 age and sex matched controls without psoriasis. The severity of psoriasis amongst subjects was assessed at base-line and follow up, in addition to gastrointestinal symptoms. Serum levels of anti-tTG were recorded for all subjects and if positive, CD diagnosis was confirmed by histology on biopsy. A total of 49% of the 218 enrolled patients with psoriasis reported one or more GI symptom, the most common being heartburn/ acid regurgitation (27%) and bloating (16%). Nine out of 218 patients with psoriasis showed positive serology for CD compared to only 1 out of the 264 controls (P=<0.05). Histology confirmed the CD diagnosis in all subjects with positivity to anti-tTG antibodies. All patients with psoriasis and CD adhered to a gluten free diet and completed the 6 month follow up. At 3 months, all 9 patients showed significant improvement in the severity of their psoriasis, this improvement was maintained at 6 months in all but 1 patient whose symptoms worsened.
This study shows a significant epidemiological association between CD and psoriasis in the Italian population. However, the small number of psoriatic patients with evidence of CD cannot be considered as definitive evidence of a causal link between the two disorders. The authors of this study hypothesise that the presence of shared genes (at-risk HLA haplotypes) often used to explain the greater prevalence of CD in several autoimmune disorders, might be present in subjects affected by psoriasis. In addition, CD-related malabsorption may affect psoriasis by causing a vitamin D deficiency status. It is well known that low levels of vitamin D pre-dispose to psoriasis, and that expose to sunlight and topical vitamin D preparations improve psoriatic lesions. Furthermore, an abnormal intestinal permeability common in untreated CD may increase the passage of different immune triggers. The fact that the majority of psoriatic subjects with positive CD serology showed complete villous atrophy is concordant with the hypotheses of vitamin D malabsorption and altered intestinal permeability to the passage of immune triggers.
Resource: Dermatology 2015 Feb 3.
Coeliac disease is increasingly associated with extraintestinal manifestations, among these, several skin disorders have been reported, with dermatitis herpetiformis being the best recognised. Several studies investigating the possible association between CD and psoriasis have been published, however results have so far proven to be inconclusive and most of the data on the effect of a gluten free diet on psoriatic skin lesions are contained within case reports.
In this prospective multicentre study, the patient records from 19 Italian primary care practices were screened for confirmed cases of psoriasis, following this 219 patients with a confirmed diagnosis of psoriasis were enrolled in to the study (111 males, 51%; mean age 54 years), and 264 age and sex matched controls without psoriasis. The severity of psoriasis amongst subjects was assessed at base-line and follow up, in addition to gastrointestinal symptoms. Serum levels of anti-tTG were recorded for all subjects and if positive, CD diagnosis was confirmed by histology on biopsy. A total of 49% of the 218 enrolled patients with psoriasis reported one or more GI symptom, the most common being heartburn/ acid regurgitation (27%) and bloating (16%). Nine out of 218 patients with psoriasis showed positive serology for CD compared to only 1 out of the 264 controls (P=<0.05). Histology confirmed the CD diagnosis in all subjects with positivity to anti-tTG antibodies. All patients with psoriasis and CD adhered to a gluten free diet and completed the 6 month follow up. At 3 months, all 9 patients showed significant improvement in the severity of their psoriasis, this improvement was maintained at 6 months in all but 1 patient whose symptoms worsened.
This study shows a significant epidemiological association between CD and psoriasis in the Italian population. However, the small number of psoriatic patients with evidence of CD cannot be considered as definitive evidence of a causal link between the two disorders. The authors of this study hypothesise that the presence of shared genes (at-risk HLA haplotypes) often used to explain the greater prevalence of CD in several autoimmune disorders, might be present in subjects affected by psoriasis. In addition, CD-related malabsorption may affect psoriasis by causing a vitamin D deficiency status. It is well known that low levels of vitamin D pre-dispose to psoriasis, and that expose to sunlight and topical vitamin D preparations improve psoriatic lesions. Furthermore, an abnormal intestinal permeability common in untreated CD may increase the passage of different immune triggers. The fact that the majority of psoriatic subjects with positive CD serology showed complete villous atrophy is concordant with the hypotheses of vitamin D malabsorption and altered intestinal permeability to the passage of immune triggers.
Resource: Dermatology 2015 Feb 3.

Coeliac Disease in Women With Infertility - A Meta-Analysis
The first indications of a link between female infertility and coeliac disease was first described over 40 years ago. However, contemporary studies have failed to reach a consensus on whether patients with infertility should be screened for coeliac disease. Many studies on this subject are small, are of poor design and/ or lack a control arm. This latest meta-analysis on the subject sought to assess whether women with infertility are at higher risk of coeliac disease. Studies excluded from the meta-analysis included those without control data, those where diagnosis of coeliac disease was based only on serology, and studies where exact data regarding the number of patients included was unavailable. 105 studies were identified as a result of the initial literature search, of these only 16 were suitable for study in detail, 11 of which did not meet the inclusion criteria, leaving just 5 for inclusion in the meta-analysis to calculate the odds of having coeliac disease in women with all cause infertility and unexplained infertility. Four further studies that had been excluded on the basis that they lacked a control arm were included for calculation of the pooled prevalence of coeliac disease in women with infertility. Studies that reported coeliac disease diagnosis based on serology but with incomplete biopsy data were all included for the calculation of pooled serological prevalence of CD in women with infertility (12 studies). The meta-analysis showed that women with infertility had a 3.5 times increased odds of having coeliac disease in comparison with that in the control population. Of 422 women with unexplained infertility across the five included studies, 14 were found to have coeliac disease, representing a 6 fold increase in the odds of having coeliac disease in comparison to the control group. When data from studies without a control arm were analysed, pooled prevalence of coeliac disease amongst 884 women with all cause infertility and 623 women with unexplained infertility was 2.3% and 3.2%, respectively. Pooled serological prevalence of coeliac disease amongst women with all-cause infertility and unexplained infertility was 1.3% and 3.1%, respectively. Micronutrient deficiencies have been suggested as a possible mechanism for infertility amongst women with coeliac disease. In this meta-analysis, 28.5% of infertility patients with coeliac disease had iron deficiency in the 4 studies that reported iron status. Among the 4 studies that reported folic acid status, none of the patients with infertility and coeliac disease were deficient in this nutrient.
Although this study makes a case for screening women with infertility for coeliac disease. The authors acknowledge that their work alone cannot be used to prove a causal relationship between coeliac disease and infertility due to the limitation of the study design of case control studies, the small number of studies available overall and the small sample sizes used within these. A recent large population-based study of infertility and coeliac disease in UK women suggested that women with coeliac disease (both before and after diagnosis) do not have a greater chance of clinically recorded fertility problems than women without coeliac disease*. However, the design of this study has it's own limitations due to reliance on primary care records, the known mis-diagnosis of coeliac disease and delay in recording this within medical records.
Resource: Dhalwani N.N, West J et al (2014) Women with celiac disease present with fertility problems no more often than women in the general population. Gastroenterology 147: 1267-74
Although this study makes a case for screening women with infertility for coeliac disease. The authors acknowledge that their work alone cannot be used to prove a causal relationship between coeliac disease and infertility due to the limitation of the study design of case control studies, the small number of studies available overall and the small sample sizes used within these. A recent large population-based study of infertility and coeliac disease in UK women suggested that women with coeliac disease (both before and after diagnosis) do not have a greater chance of clinically recorded fertility problems than women without coeliac disease*. However, the design of this study has it's own limitations due to reliance on primary care records, the known mis-diagnosis of coeliac disease and delay in recording this within medical records.
Resource: Dhalwani N.N, West J et al (2014) Women with celiac disease present with fertility problems no more often than women in the general population. Gastroenterology 147: 1267-74

Causes of death in people with coeliac disease in England compared with the general population: a competing risk analysis
Abstract
Introduction:
Quantifying excess cause-specific mortality among people with coeliac disease (CD) compared with the general population accounting for competing risks will allow accurate information to be given on risk of death from specific causes.
Method:
We identified from the Clinical Practice Research Datalink all patients with CD linked to Office for National Statistics between 1998 and 2012. We selected controls by frequency matching from the registered general practice population within 10-year age bands. We calculated the adjusted cumulative incidence (including adjustment for competing risks) and excess cumulative incidence for different causes of death up to 10 years from diagnosis.
Results:
Of the 10 825 patients with CD, 773 died within the study period. The overall mortality rate among patients with CD was 128/10 000 person years compared with 153/10 000 in controls (HR=0.94 95% CI 0.84 to 1.01). We found no overall difference in the cumulative incidence of respiratory disease, digestive disease or cancer related death among cases and controls. The adjusted cumulative incidence of death from cardiovascular deaths was slightly lower compared with those without CD diagnosis (CD 0.32% vs controls 0.41%) with a corresponding excess cumulative incidence of −0.08% (95% CI −0.13 to −0.04). However, patients with CD had 0.15% excess risk (95% CI 0.03 to 0.27) of deaths from non-Hodgkin's lymphoma from the general population baseline risk.
Conclusions:
Overall, people with CD have no major excess risk of cancer, digestive disease or respiratory disease related or cardiovascular mortality compared with the general population. These findings should be reassuring to patients with CD and clinicians managing their care.
Resource: Abdul Sultan A, et al. Gut 2014;0:1–7
Alyshah Abdul Sultan, Colin J Crooks, Tim Card, Laila J Tata, Kate M Fleming, Joe West
Introduction:
Quantifying excess cause-specific mortality among people with coeliac disease (CD) compared with the general population accounting for competing risks will allow accurate information to be given on risk of death from specific causes.
Method:
We identified from the Clinical Practice Research Datalink all patients with CD linked to Office for National Statistics between 1998 and 2012. We selected controls by frequency matching from the registered general practice population within 10-year age bands. We calculated the adjusted cumulative incidence (including adjustment for competing risks) and excess cumulative incidence for different causes of death up to 10 years from diagnosis.
Results:
Of the 10 825 patients with CD, 773 died within the study period. The overall mortality rate among patients with CD was 128/10 000 person years compared with 153/10 000 in controls (HR=0.94 95% CI 0.84 to 1.01). We found no overall difference in the cumulative incidence of respiratory disease, digestive disease or cancer related death among cases and controls. The adjusted cumulative incidence of death from cardiovascular deaths was slightly lower compared with those without CD diagnosis (CD 0.32% vs controls 0.41%) with a corresponding excess cumulative incidence of −0.08% (95% CI −0.13 to −0.04). However, patients with CD had 0.15% excess risk (95% CI 0.03 to 0.27) of deaths from non-Hodgkin's lymphoma from the general population baseline risk.
Conclusions:
Overall, people with CD have no major excess risk of cancer, digestive disease or respiratory disease related or cardiovascular mortality compared with the general population. These findings should be reassuring to patients with CD and clinicians managing their care.
Resource: Abdul Sultan A, et al. Gut 2014;0:1–7
Alyshah Abdul Sultan, Colin J Crooks, Tim Card, Laila J Tata, Kate M Fleming, Joe West

Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes
Abstract
BACKGROUND:
In the past decade, a number of population-based studies have examined the prevalence of coeliac disease in individuals with type 1 diabetes but prevalences have differed considerably.
AIM:
To examine the prevalence of coeliac disease in individuals with type 1 diabetes.
METHODS:
A systematic review of English-language articles published in PubMed Medline between 2000 and May 2014. Search terms included 'celiac disease' or 'coeliac disease' and 'diabetes mellitus'. Studies were selected with at least 100 individuals with type 1 diabetes being screened for coeliac disease where the coeliac diagnosis was later confirmed through small intestinal biopsy. Data synthesis used random-effects inverse variance-weighted models, and metaregression was used to examine heterogeneity in subgroups.
RESULTS:
A pooled analysis, based on 26,605 patients with type 1 diabetes, found a prevalence of biopsy-confirmed coeliac disease of 6.0% (95% CI = 5.0-6.9%). Heterogeneity was large (I(2) = 93.2%). The prevalence was lower in adults with type 1 diabetes (2.7%), and in mixed populations with both children and adults with type 1 diabetes (4.7%) than in children (6.2%) with type 1 diabetes (P < 0.001). Additional subgroup analyses could not explain the large variation in coeliac disease prevalence between studies.
CONCLUSION:
More than one in twenty patients with type 1 diabetes have biopsy-verified coeliac disease. This prevalence is high enough to motivate screening for coeliac disease among patients with type 1 diabetes.
Resource: Aliment Pharmacol Ther. 2014 Nov;40(10):1123-32. doi: 10.1111/apt.12973. Epub 2014 Oct 1.
BACKGROUND:
In the past decade, a number of population-based studies have examined the prevalence of coeliac disease in individuals with type 1 diabetes but prevalences have differed considerably.
AIM:
To examine the prevalence of coeliac disease in individuals with type 1 diabetes.
METHODS:
A systematic review of English-language articles published in PubMed Medline between 2000 and May 2014. Search terms included 'celiac disease' or 'coeliac disease' and 'diabetes mellitus'. Studies were selected with at least 100 individuals with type 1 diabetes being screened for coeliac disease where the coeliac diagnosis was later confirmed through small intestinal biopsy. Data synthesis used random-effects inverse variance-weighted models, and metaregression was used to examine heterogeneity in subgroups.
RESULTS:
A pooled analysis, based on 26,605 patients with type 1 diabetes, found a prevalence of biopsy-confirmed coeliac disease of 6.0% (95% CI = 5.0-6.9%). Heterogeneity was large (I(2) = 93.2%). The prevalence was lower in adults with type 1 diabetes (2.7%), and in mixed populations with both children and adults with type 1 diabetes (4.7%) than in children (6.2%) with type 1 diabetes (P < 0.001). Additional subgroup analyses could not explain the large variation in coeliac disease prevalence between studies.
CONCLUSION:
More than one in twenty patients with type 1 diabetes have biopsy-verified coeliac disease. This prevalence is high enough to motivate screening for coeliac disease among patients with type 1 diabetes.
Resource: Aliment Pharmacol Ther. 2014 Nov;40(10):1123-32. doi: 10.1111/apt.12973. Epub 2014 Oct 1.

Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity
SUMMARY
Background
Mild impairments of cognition or ‘Brain fog’ are often reported by patients with coeliac disease but the nature of these impairments has not been systematically investigated.
Aim
This longitudinal pilot study investigated relationships between cognitive function and mucosal healing in people with newly diagnosed coeliac disease commencing a gluten-free diet.
Methods
Eleven patients (8 females, 3 males), mean age 30 (range 22–39) years, were tested with a battery of cognitive tests at weeks 0, 12 and 52. Information processing efficacy, memory, visuospatial ability, motoric function and attention were tested. Small bowel biopsies were collected via routine gastroscopy at weeks 12 and 52 and were compared to baseline Marsh scores. Cognitive performance was compared to serum concentrations of tissue transglutaminase antibodies, biopsy outcomes and other biological markers.
Results
All patients had excellent adherence to the diet. Marsh scores improved significantly (P = 0.001, Friedman's test) and tissue transglutaminase antibody concentrations decreased from a mean of 58.4 at baseline to 16.8 U/mL at week 52 (P = 0.025). Four of the cognitive tests assessing verbal fluency, attention and motoric function showed significant improvement over the 12 months and strongly correlated with the Marsh scores and tissue transglutaminase antibody levels (r = 0.377–0.735; all P < 0.05). However, no meaningful patterns of correlations were found for nutritional or biochemical markers, or markers of intestinal permeability.
Conclusions
In newly diagnosed coeliac disease, cognitive performance improves with adherence to the gluten-free diet in parallel to mucosal healing. Suboptimal levels of cognition in untreated coeliac disease may affect the performance of everyday tasks.
Resource: Alimentary Pharmacology & Therapeutics Volume 40, Issue 2, pages 160–170, July 2014
Background
Mild impairments of cognition or ‘Brain fog’ are often reported by patients with coeliac disease but the nature of these impairments has not been systematically investigated.
Aim
This longitudinal pilot study investigated relationships between cognitive function and mucosal healing in people with newly diagnosed coeliac disease commencing a gluten-free diet.
Methods
Eleven patients (8 females, 3 males), mean age 30 (range 22–39) years, were tested with a battery of cognitive tests at weeks 0, 12 and 52. Information processing efficacy, memory, visuospatial ability, motoric function and attention were tested. Small bowel biopsies were collected via routine gastroscopy at weeks 12 and 52 and were compared to baseline Marsh scores. Cognitive performance was compared to serum concentrations of tissue transglutaminase antibodies, biopsy outcomes and other biological markers.
Results
All patients had excellent adherence to the diet. Marsh scores improved significantly (P = 0.001, Friedman's test) and tissue transglutaminase antibody concentrations decreased from a mean of 58.4 at baseline to 16.8 U/mL at week 52 (P = 0.025). Four of the cognitive tests assessing verbal fluency, attention and motoric function showed significant improvement over the 12 months and strongly correlated with the Marsh scores and tissue transglutaminase antibody levels (r = 0.377–0.735; all P < 0.05). However, no meaningful patterns of correlations were found for nutritional or biochemical markers, or markers of intestinal permeability.
Conclusions
In newly diagnosed coeliac disease, cognitive performance improves with adherence to the gluten-free diet in parallel to mucosal healing. Suboptimal levels of cognition in untreated coeliac disease may affect the performance of everyday tasks.
Resource: Alimentary Pharmacology & Therapeutics Volume 40, Issue 2, pages 160–170, July 2014

Managing coeliac disease in patients with diabetes
Abstract
The association between coeliac disease and type 1 diabetes has long been established. The combination of genetic susceptibility along with a potential role for gluten in the pathogenesis of autoimmunity makes defining gluten's role in type 1 diabetes extremely important. Evidence supporting the role of a gluten-free diet to improve complications associated with type 1 diabetes is not robust. However there is evidence to support improved growth, bone density and potentially the prevention of additional autoimmune diseases in patients with coeliac disease and type 1 diabetes. The gluten free diet is expensive and challenging to adhere to in people already on a modified diet. Early identification of those who have coeliac disease and would benefit from a gluten-free diet is of utmost importance to prevent complications associated with type 1 diabetes and coeliac disease.
Resource: Diabetes Obes Metab. 2014 May 9
The association between coeliac disease and type 1 diabetes has long been established. The combination of genetic susceptibility along with a potential role for gluten in the pathogenesis of autoimmunity makes defining gluten's role in type 1 diabetes extremely important. Evidence supporting the role of a gluten-free diet to improve complications associated with type 1 diabetes is not robust. However there is evidence to support improved growth, bone density and potentially the prevention of additional autoimmune diseases in patients with coeliac disease and type 1 diabetes. The gluten free diet is expensive and challenging to adhere to in people already on a modified diet. Early identification of those who have coeliac disease and would benefit from a gluten-free diet is of utmost importance to prevent complications associated with type 1 diabetes and coeliac disease.
Resource: Diabetes Obes Metab. 2014 May 9

Persistent mucosal damage and risk of fracture in celiac disease.
ABSTRACT
CONTEXT:
Celiac disease (CD) is associated with an increased fracture risk, an increase that persists after diagnosis. A significant proportion of patients with CD have persistent villous atrophy (VA) on follow-up biopsy.
OBJECTIVE:
The objective of the study was to determine whether persistent VA impacts long-term fracture risk.
DESIGN:
This was a cohort study.
SETTING AND PATIENTS:
We identified all patients in Sweden with histological evidence of CD who underwent a follow-up biopsy and compared patients with persistent VA with those with mucosal healing.
MAIN OUTCOME MEASURES:
The following were measured: 1) any fracture; 2) likely osteoporotic fracture (defined as fractures of the hip, distal forearm, thoracic and lumbar spine, or proximal humerus); and 3) hip fracture.
RESULTS:
Of 7146 patients, VA was present on follow-up biopsy in 43%. There was no significant association between persistent VA and overall fractures [hazard ratio (HR) of persistent VA compared with those with healing 0.93, 95% confidence interval (CI) 0.82-1.06] or with likely osteoporotic fractures (HR 1.11, 95% CI 0.84-1.46). Persistent VA was associated with an increased risk of hip fracture (HR 1.67, 95% CI 1.05-2.66). Hip fracture risk increased, depending on the degree of VA (HR for partial VA compared with those with healing 1.70, 95% CI 0.82-3.49, HR for subtotal/total VA compared with those with healing 2.16, 95% CI 1.06-4.41).
CONCLUSIONS:
Persistent VA on follow-up biopsy is predictive of hip fracture risk. The association between persistent VA and hip fractures, but not fractures overall, implies that thinner sc tissue and fall or trauma may be mechanisms by which persistent VA confers an increased fracture risk.
Resource: J Clin Endocrinol Metab. 2014 Feb
CONTEXT:
Celiac disease (CD) is associated with an increased fracture risk, an increase that persists after diagnosis. A significant proportion of patients with CD have persistent villous atrophy (VA) on follow-up biopsy.
OBJECTIVE:
The objective of the study was to determine whether persistent VA impacts long-term fracture risk.
DESIGN:
This was a cohort study.
SETTING AND PATIENTS:
We identified all patients in Sweden with histological evidence of CD who underwent a follow-up biopsy and compared patients with persistent VA with those with mucosal healing.
MAIN OUTCOME MEASURES:
The following were measured: 1) any fracture; 2) likely osteoporotic fracture (defined as fractures of the hip, distal forearm, thoracic and lumbar spine, or proximal humerus); and 3) hip fracture.
RESULTS:
Of 7146 patients, VA was present on follow-up biopsy in 43%. There was no significant association between persistent VA and overall fractures [hazard ratio (HR) of persistent VA compared with those with healing 0.93, 95% confidence interval (CI) 0.82-1.06] or with likely osteoporotic fractures (HR 1.11, 95% CI 0.84-1.46). Persistent VA was associated with an increased risk of hip fracture (HR 1.67, 95% CI 1.05-2.66). Hip fracture risk increased, depending on the degree of VA (HR for partial VA compared with those with healing 1.70, 95% CI 0.82-3.49, HR for subtotal/total VA compared with those with healing 2.16, 95% CI 1.06-4.41).
CONCLUSIONS:
Persistent VA on follow-up biopsy is predictive of hip fracture risk. The association between persistent VA and hip fractures, but not fractures overall, implies that thinner sc tissue and fall or trauma may be mechanisms by which persistent VA confers an increased fracture risk.
Resource: J Clin Endocrinol Metab. 2014 Feb

Association between Coeliac Disease and Psoriasis: Italian Primary Care Multicentre Study
Summary
Coeliac disease is increasingly associated with extraintest...

Coeliac Disease in Women With Infertility - A Meta-Analysis
The first indications of a link between female infertility and coeliac...

Causes of death in people with coeliac disease in England compared with the general population: a competing risk analysis
Abstract
Introduction:
Quantifying excess cause-specific mortality...

Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes
Abstract
BACKGROUND:
In the past decade, a number of population-ba...

Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity
SUMMARY
Background
Mild impairments of cognition or ‘Brain fog’ ar...

Managing coeliac disease in patients with diabetes
Abstract
The association between coeliac disease and type 1 diabete...

Persistent mucosal damage and risk of fracture in celiac disease.
ABSTRACT
CONTEXT:
Celiac disease (CD) is associated with an increa...
www.drschaer-institute.com
