Benvenuti nella nostra Clinical library
Clinical Library
46
Dieta vegetariana e celiachia
L’applicazione nello stesso individuo di due regimi alimentari diversi, quello vegetariano/vegano e quello senza glutine, pone problematiche particolari. E’ possibile seguire una dieta priva di glutine che rispetti anche le caratteristiche dello stile vegetariano o vegano in età infantile? Quali sono gli alimenti che possono entrare in uno schema che rispetti la “doppia” restrizione alimentare? Ci sono rischi nutrizionali nel seguire contemporaneamente una dieta priva di glutine e vegetariana/vegana? In questa nota cercheremo di rispondere sinteticamente a questi quesiti, particolarmente in riferimento all’età pediatrica.
Vantaggi e limiti della dieta vegetariana/vegana
Molti studi hanno messo in evidenza i possibili vantaggi degli schemi alimentari vegetariani. Gli effetti salutistici delle diete vegetariana e vegana possono essere così sintetizzati: (a) minor rischio di obesità, dislipidemia, sindrome metabolica, diabete di tipo 2, ipertensione arteriosa e cardiopatia ischemica; (b) minore incidenza di neoplasie in generale ed in particolare del tratto gastro-enterico e della prostata; (c) protezione dalla mortalità per tutte le cause e, in particolare, per patologia cardio-vascolare e neoplasie, cui corrisponde una attesa di vita più lunga di 1.5-2.4 anni della popolazione vegetariana rispetto alla quella non-vegetariana.
Per contro, occorre tenere presente i possibili rischi nutrizionali legati allo schema vegetariano/vegano. Nello schema vegetariano i principi alimentari a maggior rischio carenziale sono la vit. B12, presente quasi esclusivamente negli alimenti di origine animale, e, in misura minore, il ferro. Lo schema vegano, più ristretto, può comportare anche una relativa carenza dell’apporto calorico, proteico (per il minore valore biologico delle proteine vegetali), lipidico (soprattutto per deficit di ac. grassi della serie ω-3, docosoesaenoico = DHA e ecosopentaenoico = EPA), di zinco, calcio ed alcune vitamine (A e D). L’apporto di fibra alimentare può essere eccessivo (> 0.5 g/Kg/die) nelle diete vegetariana e vegana, il che potrebbe determinare una eccessiva diminuzione della densità calorica ed interferire negativamente con l’assorbimento dei minerali.
Aspetti nutrizionali della dieta senza glutine
Per quanto riguarda il trattamento della celiachia, la dieta senza glutine richiede notoriamente l’eliminazione di tutti i derivati del frumento, orzo e segale. Il glutine rappresenta la frazione proteica principale di questi cereali. Tuttavia, la mancanza di questa proteina nella dieta non compromette la qualità globale dell’apporto proteico, poiché il glutine presenta uno scarso valore biologico, soprattutto a causa della carenza di lisina, e può essere appropriatamente sostituito da prodotti a base di proteine di cereali non tossici (mais, riso) o altre proteine vegetali (es. legumi). Poiché il frumento rappresenta un componente primario della nostra dieta, specie di quella mediterranea, la sua eliminazione può comunque determinare una relativa carenza di altri nutrienti, soprattutto fibre, calcio, ferro e folati. A tali carenze, peraltro marginali, può contribuire anche il malassorbimento intestinale presente nella celiachia prima della diagnosi.
Consigli per i celiaci a dieta vegetariana/vegana
Premesso che non vi sono motivi per negare uno schema vegetariano/vegano ad un bambino (o adulto) che debba seguire una dieta senza glutine, va da sé che il duplice regime dietetico richieda alcune attenzioni particolari, che riassumeremo di seguito.
Nel celiaco a dieta vegana è importante assicurare un adeguato apporto di alimenti ad elevata densità calorica quali cereali privi di glutine e pseudo-cereali, marmellate, frutta secca ed olio extravergine di oliva. Sempre nella dieta vegana, in considerazione della ridotta digeribilità delle proteine vegetali, è consigliabile aumentare l’apporto giornaliero proteico del 30-35% fino a due anni, 20-30% fino a 6 anni e 15% dopo i 6 anni, rispetto ai valori consigliati per i non-vegetariani. Le maggiori fonti proteiche di origine vegetale sono i legumi, i cereali (ovviamente senza glutine per il celiaco). Le migliori fonti alimentari di B12 per i vegetariani/vegani sono rappresentate dagli alimenti addizionati, es. le formule a base di soia. E’ inoltre consigliabile un apporto maggiorato dell’80% di ferro, sia nei vegetariani che vegani, a causa della minore biodisponibilità del ferro presente nei vegetali. Da tenere presente che gli alimenti ricchi di vit. C favoriscono l’assorbimento del ferro, mentre alcuni componenti vegetali (fitati, tannini e fibre) lo inibiscono. L’uso di alimenti fortificati in ferro può essere consigliabile durante le fasi di rapida crescita. Anche per lo zinco si pone un problema di minore biodisponibilità soprattutto in caso di elevato apporto di fitati, di cui sono ricchi i cereali integrali ed i legumi. Pertanto è consigliabile un apporto alimentare di zinco superiore del 50% rispetto al normale. Gli alimenti più ricchi di zinco sono i legumi, la frutta secca, il formaggio e i prodotti a base di soia fermentata. L’apporto di calcio è adeguato nella dieta vegetariana, poiché il latte e derivati ne contengono elevate quantità, mentre può risultare inadeguato nella dieta vegana. E’ opportuno pertanto utilizzare alimenti addizionati di calcio, come prodotti di soia, o naturalmente ricchi di questo minerale come le verdure a foglia, i cavoli e le mandorle.
Alcuni cibi ad elevato contenuto di grassi come le noci, i semi oleaginosi (girasole, zucca, sesamo, lino, papavero), avocado, prodotti di soia e oli vegetali, rivestono un ruolo importante nella dieta vegana. E’ importante che le diete vegane includano fonti di ac. alfa-linolenico (ALA, ω-3) quali i semi di lino macinati, le noci ed i prodotti derivati dalla soia, e oli a ridotto contenuto di ac. linoleico (ω-6), quale l’olio extravergine di oliva.
Sebbene una modesta esposizione solare nei bambini con pelle chiara sia sufficiente a prevenire la carenza di vit. D, uno scarso apporto alimentare può verificarsi nella dieta vegana. Il problema può essere risolto mediante assunzione di alimenti integrati (formule a base di soia o di riso) o supplementazione farmacologica. Una dieta priva di carne è povera di vit. A, ma la carenza può essere prevenuta grazie al consumo di alimenti ricchi di carotene (pro-vitamina A), di cui sono ricchi la frutta, le verdure di colore arancio o giallo e quelle a foglia.
Il problema della contaminazione di glutine
Nel celiaco rimane fondamentale l’esigenza di assicurare l’assenza di contaminazione di glutine negli alimenti ingeriti. Purtroppo alcuni degli alimenti che trovano impiego negli schemi vegetariani/vegani, quali soia, legumi e semi oleaginosi, presentano un elevato rischio di contaminazione di glutine. E’ bene pertanto scegliere questi prodotti tra quelli che riportano sulla confezione la certificazione dell’assenza di glutine.
Conclusioni
La scelta vegetariana o vegana rappresenta una opzione valida anche per il bambino e pe l’adulto celiaco in trattamento con dieta priva di glutine. Le scelte alimentari devono essere pianificate tenendo presente sia le esigenze nutrizionali brevemente discusse in questa nota che quella di evitare la contaminazione di glutine della dieta.

Diagnosi di sensibilità al glutine non celiaca (SGNC) in pazienti con sintomi funzionali gastrointestinali: risultati di uno studio multicentrico, randomizzato, in doppio cieco contro placebo con provocazione con glutine.
L’obiettivo di questo studio clinico era individuare in maniera affidabile pazienti con SGNC all’interno di una coorte di pazienti, che registrava un miglioramento dei sintomi gastrointestinali in assenza di glutine, attraverso uno specifico protocollo diagnostico eseguito in doppio cieco con provocazione con glutine contro placebo con crossover. Lo studio clinico è stato condotto in 15 centri ambulatoriali italiani di gastroenterologia. Sono stati inclusi in questo studio clinico 140 pazienti adulti, che consultano regolarmente ambulatori gastroenterologici e soddisfano i criteri ROMA III per disturbi funzionali di stomaco e intestino. Tutti i pazienti assumevano alimenti contenenti glutine, risultavano negativi ai test degli anticorpi di classe IgA delle immunoglobuline anti Tissue-Transglutaminase (IgA-tTGA) e all’allergia al grano IgE mediata e presentavano un tenore totale di IgA normale. In pazienti con forte sospetto clinico di celiachia è stata eseguita inoltre una biopsia duodenale, al fine di escludere i pazienti con celiachia sieronegativa.
La fase 1 dello studio clinico ha esaminato la reazione dei pazienti all’assenza di glutine. Il primo passo è stata la valutazione dei sintomi della malattia e della qualità della vita in relazione alla salute, mediante Scale Analogiche Visive (VAS), scala da 1 a 10, e il questionario sullo stato di salute Short Form 36 (SF36). Al termine di questa valutazione, è stata introdotta un’alimentazione priva di glutine della durata di 3 settimane. I pazienti hanno ricevuto ampia consulenza e supporto da parte di un esperto dell’alimentazione. Alla fine della fase 1, i pazienti hanno compilato nuovamente le scale VAS e il questionario SF36. I pazienti che hanno indicato un miglioramento significativo dello stato di salute (VAS ≥ 3, n = 101) sono stati considerati “responder al glutine” e sono stati inseriti nella seconda fase dello studio clinico.
Questi pazienti responder sono stati esortati a continuare ad alimentarsi rigorosamente senza glutine nella fase 2 (provocazione con glutine in doppio cieco contro placebo con crossover). 98 pazienti sono stati ammessi a questa fase dello studio clinico (3 pazienti hanno rinunciato a proseguire la partecipazione per paura di recidive sintomatiche se sottoposti a provocazione con glutine). I pazienti sono stati suddivisi in due gruppi randomizzati e per 7 giorni hanno assunto 5,6 g/giorno di glutine (capsule con glutine trattato, pari a 80 g di pasta secca) o placebo (capsule con amido di riso). Prima del crossover si è svolto un periodo di sospensione (wash-out) di 7 giorni. Di conseguenza, la fase 2 dello studio clinico ha avuto una durata complessiva di 21 giorni (durante questo periodo, i pazienti hanno continuato ad alimentarsi senza glutine). Al termine di ogni fase (provocazione con glutine/placebo, fase di sospensione e crossover), vale a dire ogni 7 giorni, i pazienti hanno compilato nuovamente le scale VAS e il questionario SF36. Nel complesso, i pazienti hanno riferito peggioramenti dello stato di salute più marcati sotto provocazione con glutine che sotto placebo (p = 0,05). 28 dei pazienti randomizzati hanno reagito ‘positivamente’ alla provocazione con glutine in doppio cieco contro placebo con crossover (in altre parole, soffrivano di una recidiva sintomatica sotto assunzione di glutine) e 69 pazienti si sono dimostrati negativi alla provocazione con glutine in doppio cieco contro placebo con crossover (vale a dire, nessuna recidiva sintomatica sotto assunzione di glutine). Non è stata constatata nessuna correlazione tra fattori demografici, clinici o biochimici e la reazione alla provocazione con glutine. Tra i pazienti identificati come ‘positivi’, la sequenza in cui erano state assunte le capsule di glutine e di placebo non sembra avere alcun effetto significativo. Da notare che 14 dei 28 pazienti ‘positivi’ sono stati identificati anche come responder al placebo. Come prevedibile, questo risultato evidenzia un effetto placebo significativo.
Nel complesso, il 14% dei 98 “responder al glutine” randomizzati hanno registrato una recidiva sintomatica durante la provocazione con glutine in cieco, controllata con placebo (senza risposta contemporanea al placebo) e, di conseguenza, è stato possibile identificarli come pazienti con SGNC. Questo risultato conferma che l’assunzione di glutine in un sottogruppo di pazienti con disturbi funzionali dell’intestino può provocare sintomi gastrointestinali. Si tratta del primo studio clinico a indagare e valutare l’efficacia del protocollo diagnostico in due fasi “Diagnosis of NCGS: The Salerno Experts’ Criteria”1 nella prassi clinica. L’elevato numero di pazienti che ha reagito all’assenza di glutine (75%), ma non alla provocazione con glutine dopo l’assenza di glutine, è degna di nota. Presumibilmente, questa discrepanza è riconducibile in parte a un possibile effetto placebo. Molti pazienti, tuttavia, potrebbero reagire anche ad altre sostanze aspecifiche nel frumento, per es. ATIs o FODMAPs.
1 Catassi C, Elli L, Bonaz B et al. Diagnosis of Non-coeliac Gluten Sensitivity (NCGS): The Salerno Experts Criteria. Nutrients 2015; 7: 4966-4977.
Elli L, Tomba C, Branchi R et al
Resource: Nutrients 2016; 8: 84; doi:10.3390/nu8020084

L’impiego dell’avena nella dieta senza glutine
La celiachia è una patologia autoimmunitaria indotta dal glutine, complesso proteico presente nel frumento, orzo e segale, caratterizzata dalla presenza di atrofia della mucosa intestinale, autoanticorpi circolanti e sintomi intestinali ed extraintestinali. La frequenza della celiachia nella popolazione generale è pari a circa l’1 %. Il trattamento della celiachia, e di altri disturbi indotti dal glutine quali la sensibilità al glutine non celiaca (NCGS), si basa notoriamente sulla esclusione del frumento, orzo e segale, mentre sono permessi i cereali privi di glutine, quali il riso ed il mais, e tutti gli altri alimenti naturali.
L’avena era stata originariamente esclusa nel trattamento dietetico della celiachia, sebbene mancassero studi scientifici sulla potenziale tossicità, per il celiaco, di questo importante alimento. A partire dagli anni ’90 del secolo scorso, numerosi lavori scientifici hanno invece dimostrato, sulla base di dati clinici, immunologici ed istologici, che l’avena non contaminata dal glutine non solo è ben tollerata, ma può anche migliorare la qualità nutrizionale della dieta senza glutine. Ormai da molti anni, prodotti a base di avena non contaminata sono ampiamente consumati da parte di pazienti celiaci nei paesi del NordEuropa, quali il Regno Unito, l’Irlanda e la Finlandia, nei quali questo cereale è sempre stato un alimento basilare per tutta la popolazione.
In Italia l’impiego dell’avena nell’alimentazione umana è tradizionalmente poco diffuso. Questo riscontro spiega verosimilmente sia il minore interesse al “recupero” di questo cereale nella mensa del celiaco, che l’assenza nel mercato italiana, a tutt’oggi, di prodotti a base di avena non contaminata per il trattamento della celiachia. Tuttavia, considerazioni di tipo nutrizionale-salutistico, oltre che la comprovata sicurezza di questo cereale, suggeriscono che, anche nel nostro Paese, la comunità delle persone intolleranti al glutine possa trarre benefici dall’impiego alimentare dell’avena.
In questo documento verranno analizzati i dati clinici, immunologici e nutrizionali, sulla base dei quali è stata dimostrata la sicurezza e l’efficacia dell’impiego dell’avena nel trattamento della celiachia.
Per approfondire l'argomento clicca qui.

Da oggi a tavola c’è … una nuova opportunità: l’AVENA
L’avena – tra gli alimenti ricchi in carboidrati - si pone su di un piano privilegiato poiché permette di allungare l’elenco dei cereali permessi a coloro che vivono reazioni avverse al glutine e al contempo, rappresenta un vero e proprio scrigno di salute grazie alla sua ricchezza in nutrienti.
Da un punto di vista botanico e anatomico l’Avena sativa, , è una pianticella erbacea annuale, della famiglia delle Graminacee. Ha fiori piccoli, quasi insignificanti, riuniti in una pannocchietta ricadente, mentre i frutti, che entrano nell’alimentazione umana sono detti cariossidi ed hanno una forma ellittica e allungata con un caratteristico solco laterale.
Perché l’avena è così importante? Quali sono i suoi effetti positivi? Come può essere utilizzata in cucina? Ci sono controindicazioni?
Prima di dare risposta alle singole domande mi preme riportare con chiarezza quanto ad oggi la comunità scientifica asserisce sull’utilizzo dell’avena da parte di coloro che vivono reazioni avverse al glutine, inclusi i celiaci.
La maggior parte dei celiaci può inserire l'avena nella propria dieta senza effetti negativi per la salute. Il Board Scientifico di AIC, pertanto, suggerisce il consumo di avena solo per quei prodotti ‘a base di’ o ‘contenenti’ avena presenti nel Registro Nazionale dei prodotti senza glutine del Ministero della Salute, che garantisce sull’idoneità dell’avena impiegata. Allo scopo di monitorare eventuali effetti legati all’introduzione dell’avena, si consiglia inoltre che tali prodotti vengano inizialmente somministrati a pazienti in completa remissione e che stiano seguendo una dieta senza glutine che abbia escluso anche l’avena. (Fonte: www.celiachia.it - ultimo accesso 12 ottobre 2015)
Perché l’avena è così importante?
Questa la carta d’identità dell’ avena: 100 g di avena contengono: 389 kcal.
- Carboidrati 66.27 g
- Grassi 06.90 g
- Proteine 16.89 g
- Fibra alimentare 10.6 g
- Colesterolo 0.0 mg
- Tiamina 0.8 mg
- Folati 56.0 mcg
- Acido pantotenico 01.3 mg
- Manganese 04.9 mg
- Fosforo 523 mg
- Magnesio 177 mg
- Rame 0.6 mg
- Ferro 4.7 mg
- Zinco 4.0 mg
Dall’analisi nutrizionale emerge immediatamente che l’avena tra tutti i cereali detiene il primato di alimento più ricco in proteine e di grassi ma altre peculiarità rendono l’avena un cereale così tanto importante per la salute umana.
Le proteine non contengono la frazione tossica per i celiaci (globuline 50% - avenine 4-14% mentre albumine e glutenine 9-20% e 21-27% rispettivamente) e garantiscono un elevato valore biologico, determinato dalla discreta quantità di metionina, cisteina e di lisina, aminoacido essenziale nella sintesi proteica.
Tra i grassi ci sono importanti sottolineature come il discreto contenuto di acido linoleico, essenziale per la sintesi delle prostaglandine. La frazione lipidica si completa inoltre con trigliceridi, acidi grassi liberi mono e digliceridi, steroli, fosfolipidi e componenti biologicamente attivi quali vitamina E e carotenoidi.
Tra i carboidrati esigua è la componente di zuccheri semplici mentre è importante la frazione dei carboidrati complessi quali fibra alimentare ed amido. La fibra alimentare in particolare è rappresentata da β-glucano, lignina, cellulosa ed emicellulosa. Mentre l’amido dell’avena -sempre composto dalle due frazioni amilosio e amilopectina come negli altri cereali ma che in questo caso combinandosi in diversa percentuale conferiscono una maggiore elasticità e diversa viscosità rispetto all’amido di mais e di frumento.
Infine l’ avena, oltre alla ricchezza in minerali e vitamine, regala al nostro organismo importanti composti con elevato potere antiossidante: phytochemicals quali tocoli, acidi fenolici, flavonoidi, steroli, acido fitico e avenantramidi.
Da questa ricca e peculiare composizione chimica emergono importanti e positivi effetti sulla salute dell’uomo sia nel trattamento che nella prevenzione di alcune patologie cronico-degenerative.
Quali sono i suoi effetti positivi?
Sono sicuramente numerosi, ma l’attenzione sarà posta su quelli che sembrano essere più ricercati per la popolazione di coloro che vivono reazioni avverse al glutine in quanto la loro alimentazione è sbilanciata in zuccheri, grassi, fibra alimentare e phytocomposti.
L’introduzione dell’avena coadiuva il mantenimento del buon funzionamento dei nostri laboratori; ad esempio, l’apporto e la qualità della fibra alimentare presente nell’avena permette il controllo del senso di sazietà e grazie al suo potere igroscopico consente il controllo dell’appetito e della stipsi. La fibra entra nella mediazione di numerosi processi della digestione riducendo naturalmente l’assorbimento dei lipidi fino a favorire la proliferazione dei microorganismi con potere anti-ossidante e anti-infiammatorio e dei batteri che partecipano alla sintesi delle vitamine.
L’associazione della fibra alimentare (in particolare β-glucano) con la presenza di grassi polinsaturi e di phytocomposti permette inoltre, il controllo dei lipidi circolanti, soprattutto colesterolo, il cui eccesso sappiamo essere fattore di rischio per le patologie cardio-vascolari, ictus e cancro oltre ad accelerare la formazione di placche lipidiche nei vasi.
Sono sempre i β-glucani a giocare un ruolo potenziante nei confronti del sistema immunitario. Hanno infatti un ruolo preventivo e di difesa in quanto capaci di ridurre l’infiammazione e di conseguenza l’insorgenza di patologie che derivano da infiammazioni croniche quali il tumore.
Un ultimo effetto positivo dei β-glucani, non certo per importanza quanto piuttosto perché ancora in fase di sperimentazione, è quello di controllare la glicemia poiché sembra essere in grado di simulare l’effetto dell’insulina. Si aggiunge anche il fatto che tutti gli alimenti a base di avena hanno un basso IG e riducono l’innalzamento del picco post-prandiale proprio grazie alla elevata quantità di fibra alimentare.
Come può essere utilizzata in cucina?
L’avena in cucina è davvero molto versatile infatti in base ai diversi tipi di lavorazione si possono ottenere:
- Chicchi: non schiacciati , ottimi per la prima colazione.
- Fiocchi: grazie al processo di trafilatura si ottengono delle croccanti sfogliette.
- Crusca: la si può mangiare tal quale o aggiungere alle preparazioni.
- Farina: ricavata da macinazione e utilizzata come materia prima per prodotti dolci e/o salati.
Ci sono controindicazioni?
Abbiamo presentato fin qui la ricchezza nutrizionale e discusso gli effetti positivi di un alimento e non certo di un farmaco pertanto parlare di contro-indicazioni sembra inappropriato.
Al tempo stesso possiamo affermare che come in ogni alimento, anche nell’avena possono essere presenti componenti che innescano reazioni avverse variabili da soggetto-a-soggetto.

Revisione sistematica con meta-analisi: nutrizione nella prima infanzia e malattia celiaca – aggiornata al 2015.
Allattamento al seno e MC
Lo studio PREVENT CD ha dimostrato che l’allattamento al seno esclusivo o di altro tipo non influisce in maniera significativa sullo sviluppo della MC, mentre lo studio CELIPREV ha evidenziato che l’allattamento al seno dei bambini che avevano sviluppato la MC si è protratto per un lasso di tempo simile a quello dei bambini che non avevano sviluppato la patologia. I risultati aggregati di cinque studi osservazionali confermano questa evidenza, anche se tra gli studi clinici si riscontra una notevole eterogeneità.
Introduzione del glutine durante l’allattamento al seno e MC
Né lo studio PREVENT CD né il CELIPREV sono riusciti a dimostrare che l’introduzione del glutine durante l’allattamento al seno influisca sul rischio di sviluppare la MC. I risultati aggregati di sette studi osservazionali confermano questa evidenza. Tra gli studi clinici si riscontra una notevole eterogeneità.
Epoca di introduzione del glutine e MC
Lo studio PREVENT CD ha confermato che l’introduzione del glutine a 16 settimane invece che a 24 settimane non ha comportato un incremento del rischio di MC a 3 anni. Nello studio CELIPREV, l’introduzione del glutine a 6 mesi vs. 12 mesi ha determinato un incremento del rischio di MC a 2 anni ma non a 5 anni (obiettivo primario). Gli studi clinici non hanno riscontrato alcuna differenza significativa tra introduzione precoce e tardiva del glutine in termini di prevalenza della MC, con l’eccezione dello studio ETICS che ha evidenziato un aumento significativo del rischio nei bambini esposti al glutine a partire dai 6 mesi di vita rispetto a quelli esposti a piccole quantità tra i 4 e i 6 mesi. Tra gli studi clinici si riscontra una notevole eterogeneità.
Quantità di glutine nello svezzamento e MC
Lo studio PREVENT CD ha dimostrato che modeste assunzioni giornaliere di glutine non avevano avuto effetti sullo sviluppo della MC; i dati che hanno permesso di giungere a questo risultato, tuttavia, erano disponibili solo per un sottogruppo di partecipanti: non essendo un obiettivo di studio primario, le quantità di glutine a cui erano stati esposti i singoli gruppi non erano state comparate. Solo uno degli studi osservazionali ha analizzato il quantitativo di glutine assunto dai bambini; nei bambini di età inferiore a due anni il rischio di MC era maggiore se il glutine veniva introdotto in grandi quantità piuttosto che in piccole o medie quantità, tuttavia l’assunzione di glutine non era quantificata in termini di grammi al giorno e non si riscontrava alcun effetto sui bambini più grandi.
Tipo di glutine e MC
Non è stata condotta alcuna sperimentazione interventistica volta ad appurare gli effetti di un certo tipo di glutine sul rischio di sviluppare la MC. I cibi erano catalogati solo come “cibi solidi” (pane, biscotti, budino, pasta) o come “alimenti di proseguimento contenenti glutine”.
Glutine durante il periodo di lattazione e MC
Lo studio PREVENT CD ha dimostrato che la dieta materna in gravidanza e allattamento non influisce sul rischio che il feto sviluppi la MC.
Gli autori della presente revisione sono giunti alla conclusione che l’epoca di introduzione del glutine e la durata o il proseguimento dell’allattamento al seno in fase di introduzione del glutine non influiscono sul rischio di MC, perlomeno non nei tempi degli studi. I dati sul quantitativo di glutine in svezzamento in relazione al rischio di MC non consentono di trarre conclusioni decisive. Le informazioni relative al tipo di glutine usato durante lo svezzamento sono carenti, ma non sembra che ciò rappresenti un fattore di rischio. A conoscenza degli autori non sono stati condotti studi volti a valutare l’effetto delle pratiche di nutrizione sul rischio di MC tra i bambini portatori di tipi diversi di HLA, anche se l’elevata prevalenza di MC in bambini piccoli omozigoti per la HLA 2.5 suggerisce che le pratiche di nutrizione giochino un ruolo minimo, se non nullo, nei bambini con un’alta predisposizione genetica. Questa revisione sistematica aggiornata non conferma i risultati di precedenti studi osservazionali che suggerivano una correlazione tra l’introduzione del glutine in un dato periodo, l’allattamento al seno e il rischio di MC. Si impone dunque un aggiornamento delle raccomandazioni europee in questo ambito.
Resource: Aliment Pharmacol and Ther 2015; 41 1038-1054

Non-celiac gluten sensitivity has narrowed the spectrum of irritable bowel syndrome: A double-blind randomized placebo-controlled trial
148 adult patients with IBS and fulfilling the Rome III criteria were initially recruited from a private gastroenterology clinic in Tehran. Patients excluded from the study included those with a diagnosis of coeliac disease or inflammatory bowel disease, those who were already following or had previously followed a wheat or gluten free diet, those using drugs for depression/ anxiety, those using non-steroidal anti-inflammatory drugs, and those with abnormal electrolytes levels or thyroid function tests. The resulting patient sample eligible to commence a gluten free diet was 102, of these 22 patients withdrew from the study on the basis that the diet was too difficult to follow. A further 8 patients found relief from a gluten free diet but decided to withdraw as they were unprepared to follow the diet strictly. The remaining 72 patients completed the study (19 males and 53 females). Patients were randomly assigned to the gluten group (35 patients; 6 males and 29 females; mean age 44.5 +/- 10 years) or the placebo group (37 patients; 13 males and 24 females; mean age 43.2 +/- 17 years). There was no statistical difference between the 2 groups in terms of IBS sub-type. Patients completed a visual analogue symptom questionnaire at baseline to investigate primary outcomes including bloating, abdominal pain, defecation satisfaction, nausea, fatigue, and overall symptoms. Serum markers were measured for IgA anti-tTG, IgA anti-gliadin and IgG anti-gliadin antibodies, HLA typing was also performed for all patients.
All 72 patients then commenced a 6 week gluten free diet with weekly assessment by a dietitian to monitor and support compliance. After 6 weeks the gluten group were each asked to consume 100g/day (2 x 50g doses) of gluten powder (FODMAP free), the placebo group were asked to consume 100g/ day of gluten-free powder (rice flour, corn starch and glucose). Powders were mixed with warm water and consumed with breakfast and tea each day for a further 6 weeks. All patients remained on a gluten free diet for this second 6 week phase of the study.
After the 6 week challenge phase, symptoms were controlled in 25.7% of the gluten-containing group v’s 83.8% in the placebo group, indicating that 26 of the 35 patients in the gluten group became symptomatic on gluten challenge. Symptoms increased significantly in the gluten group, particularly for bloating and abdominal pain within 1 week of commencing the gluten challenge. No statistical difference was noted in serum antibody levels after gluten challenge. The authors of this study conclude that there is growing evidence that gastrointestinal symptoms in many IBS patients may be improved after gluten exclusion and differentiation between patients with IBS responsive to FODMAP restriction and those with Non-coeliac gluten sensitivity is important. This is particularly the case since evidence is emerging that a low FODMAP diet may reduce numbers of favourable bacteria in the gut (1,2). The authors went on to suggest a potential algorithm for the management of IBS patients fulfilling Rome III criteria that first implements a 6 week gluten free diet and goes on to recommend further carbohydrate restriction only in non-responders.
1. Staudacher HM, Lomer MC, Anderson JL et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr 2012; 142: 1510-1518
2. Halmos EP, Christopherson CT, Bird AR et al. Diet that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015; 64:93-100
Resource: Nutrients June 2015; 7:4542-4554
Shahbazkhani B, Sadeghi A, Malekzadeh R et al

Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria
Non-Celiac Gluten Sensitivity (NCGS) is a syndrome characterized by intestinal and extra-intestinal symptoms related to the ingestion of gluten-containing food, in subjects that are not affected by either celiac disease or wheat allergy. Given the lack of a NCGS biomarker, there is the need for standardizing the procedure leading to the diagnosis confirmation. In this paper we report experts’ recommendations on how the diagnostic protocol should be performed for the confirmation of NCGS. A full diagnostic procedure should assess the clinical response to the gluten-free diet (GFD) and measure the effect of a gluten challenge after a period of treatment with the GFD. The clinical evaluation is performed using a self-administered instrument incorporating a modified version of the Gastrointestinal Symptom Rating Scale. The patient identifies one to three main symptoms that are quantitatively assessed using a Numerical Rating Scale with a score ranging from 1 to 10. The double-blind placebo-controlled gluten challenge (8 g/day) includes a one-week challenge followed by a one-week washout of strict GFD and by the crossover to the second one-week challenge. The vehicle should contain cooked, homogeneously distributed gluten. At least a variation of 30% of one to three main symptoms between the gluten and the placebo challenge should be detected to discriminate a positive from a negative result. The guidelines provided in this paper will help the clinician to reach a firm and positive diagnosis of NCGS and facilitate the comparisons of different studies, if adopted internationally.
Resource: Nutrients 2015, 7(6), 4966-4977; doi:10.3390/nu7064966
Carlo Catassi, Luca Elli, Bruno Bonaz, Gerd Bouma, Antonio Carroccio, Gemma Castillejo, Christophe Cellier, Fernanda Cristofori, Laura de Magistris, Jernej Dolinsek, Walburga Dieterich, Ruggiero Francavilla, Marios Hadjivassiliou, Wolfgang Holtmeier, Ute Körner, Dan A. Leffler,Knut E. A. Lundin, Giuseppe Mazzarella, Chris J. Mulder, Nicoletta Pellegrini, Kamran Rostami, David Sanders, Gry Irene Skodje, Detlef Schuppan, Reiner Ullrich, Umberto Volta, Marianne Williams, Victor F. Zevallos, Yurdagül Zopf and Alessio Fasano

Small Amounts of Gluten in Subjects With Suspected Nonceliac Gluten Sensitivity: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial.
BACKGROUND & AIMS:
There is debate over the existence of nonceliac gluten sensitivity (NCGS) intestinal and extraintestinal symptoms in response to ingestion of gluten-containing foods by people without celiac disease or wheat allergy. We performed a randomized, double-blind, placebo-controlled, cross-over trial to determine the effects of administration of low doses of gluten to subjects with suspected NCGS.
METHODS:
We enrolled 61 adults without celiac disease or a wheat allergy who believed ingestion of gluten-containing food to be the cause of their intestinal and extraintestinal symptoms. Participants were assigned randomly to groups given either 4.375 g/day gluten or rice starch (placebo) for 1 week, each via gastrosoluble capsules. After a 1-week gluten-free diet, participants crossed over to the other group. The primary outcome was the change in overall (intestinal and extraintestinal) symptoms, determined by established scoring systems, between gluten and placebo intake. A secondary outcome was the change in individual symptom scores between gluten vs placebo.
RESULTS:
According to the per-protocol analysis of data from the 59 patients who completed the trial, intake of gluten significantly increased overall symptoms compared with placebo (P = .034). Abdominal bloating (P = .040) and pain (P = .047), among the intestinal symptoms, and foggy mind (P = .019), depression (P = .020), and aphthous stomatitis (P = .025), among the extraintestinal symptoms, were significantly more severe when subjects received gluten than placebo.
CONCLUSIONS:
In a cross-over trial of subjects with suspected NCGS, the severity of overall symptoms increased significantly during 1 week of intake of small amounts of gluten, compared with placebo.
Resource: Clin Gastroenterol Hepatol. 2015 Sep;13(9):1604-1612.
Di Sabatino A, Volta U, Salvatore C, Biancheri P, Caio G, De Giorgio R, Di Stefano M, Corazza GR.

Clinical effectiveness and economic costs of group versus one-to-one education for short-chain fermentable carbohydrate restriction (low FODMAP diet) in the management of irritable bowel syndrome.
Patients were considered suitable for inclusion if they had been diagnosed with IBS by their primary care physician or gastroenterologist using NICE criteria, had organic disease ruled out and had been referred for dietetic advice. These patients were first offered a telephone consultation which lasted up to 15 minutes during which they were assessed for suitability to join a group session (brief assessment including symptoms, medical history and dietary intake). Those considered suitable were given a group appointment. Patients considered unsuitable for group education included those with atypical symptoms, complex health issues or language barriers, these patients were offered one-to-one dietetic appointments. Symptom type and severity, alongside stool frequency and form were assessed at base-line and at follow up by means of a questionnaire. Patients who did not attend follow-up were excluded from the study. Patients attending group sessions also completed additional questionnaires to assess the acceptability of group education.
The initial appointment for group sessions lasted approximately 90 minutes and included up to 12 patients. One-to-one sessions lasted approximately 1 hour. Follow up appointments (booked a minimum of 6 weeks later) were delivered using the same educational approach as at baseline (group or one-to-one). Data from 263 patients attending group sessions was compared to data from 101 patients attending one-to-one education. There was no significant difference between groups for IBS subtype at baseline. There was a significant increase in the proportion of patients reporting ‘yes’ to the global symptom question (‘do you currently have satisfactory relief of your gut symptoms’) following low FODMAP advice via both methods of education (18% at baseline versus 54% at follow-up for group education, and 5% at baseline versus 60% at follow-up for one-to-one education), with no difference at follow-up between the 2 methods. Patients had a significant decrease in composite symptom score following low FODMAP dietary advice, composite scores were not significantly different between the two methods of education at follow-up.
At follow-up, 93% of patients reported adherence to the diet at least 50% of the time. The majority of patients (87%) reported that the written information provided was easy to understand and 49% found the diet easy to follow. Following group education, 36% of patients would have preferred one-to-one education, with the remainder preferring group education (22%) or expressing no preference (42%). Most (81%) felt that group education had provided sufficient information to manage their symptoms without further advice. Unfortunately, non-attendance data was not reported in this study as the primary focus was symptom response (requiring those who did not attend follow up to be excluded from the evaluation). The cost of providing low FODMAP dietary advice was calculated as £67.19 per patient for group education (assuming 12 patients per group) and £139.20 per patient for one-to-one education. The cost of a QALY gain for this group pathway was well below the £20,000 to £30,000 threshold for UK healthcare costs.
In conclusion, this study shows that dietitian-led FODMAP group education is clinically effective and the costs associated with a FODMAP group pathway are worthy of further investigation.
Resource: Journal of Human Nutrition and Dietetics (2015). 10.1111/jhn.12318
Whigham L, Joyce T, Harper G et al

Systematic review: non coeliac gluten sensitivity
Non coeliac gluten sensitivity (NCGS) is a controversial emerging disorder. Despite reported symptoms related to the ingestion of gluten, NCGS remains a diagnosis based on the exclusion of coeliac disease, given the absence of reliable biomarkers.
Aim
To evaluate the prevalence, diagnostic exclusion of coeliac disease and the efficacy of a gluten-free diet (GFD) for NCGS patients.
Methods
A PubMed search was performed up to December 2014. According to consensus diagnostic criteria, NCGS was defined as self-reported gluten intolerance, negative coeliac serology and absence of villous atrophy. Studies evaluating the impact of a GFD on patients with irritable bowel syndrome (IBS) were also included.
Results
Prevalence rates of NCGS (0.5–13%) differed widely. Seventeen studies, including 1561 patients (26 children), met the inclusion criteria for NCGS. HLA haplotypes could not be linked to histology [normal or lymphocytic enteritis (LE)] in 1123 NCGS patients. HLADQ2/DQ8 haplotypes were present in 44% of NCGS patients. After advanced diagnostic techniques in 189 NCGS patients combining LE and HLADQ2/DQ8 haplotypes, 39 (20%) were reclassified as coeliac disease. There was a higher than expected family history of coeliac disease and autoimmune disorders in NCGS patients. A GFD resulted in variable results for variable, but significantly improved stool frequency in HLADQ2 positive diarrhoea-predominant IBS patients.
Conclusions
Prevalence rates for NCGS are extremely variable. A subset of NCGS patients might belong in the so-called ‘coeliac-lite’ disease. The benefit of a GFD for NCGS patients is currently controversial. HLADQ2 positive diarrhoea- type IBS patients might gain symptom improvement from a GFD.
Resource: Aliment Pharmacol Ther. 2015 May;41(9):807-20. doi: 10.1111/apt.13155. Epub 2015 Mar 6.
Molina-Infante J, Santolaria S, Sanders DS, Fernández-Bañares F.

A Study Evaluating the Bidirectional Relationship Between Inflammatory Bowel Disease and Self-reported Non-coeliac Gluten Sensitivity.
No significant difference was found between patients with ulcerative colitis and Crohn’s disease in terms of prevalence on SR-NCGS and gluten free diet usage. However, IBD patients with SR-NCGS were significantly more likely to have stricturing disease (p=0.046) and more active disease (p=0.002) as measured by the Crohn’s Disease Activity Index score. This indicates that the presence of SR-NCGS may be a marker for clinicians of underlying severe/ structuring disease. The mechanism for the potential therapeutic effect of a gluten free diet in IBD is not well understood. The volume/ physical properties and ‘higher residue’ nature of gluten-containing foods may lead patients to avoid such products. It is also possible that an unidentified immunological mechanism may contribute to the relief experienced by patients choosing to reduce or eliminate their intake of gluten. The absence of diagnostic markers for NCGS mean it is unclear whether individuals with IBD who self-report gluten sensitivity are sensitive the gluten or other components of wheat such as mannans, amylase-trypsin inhibitors or FODMAPs. Further studies are needed to understand whether a gluten-free diet, or indeed a wider wheat free diet may be a valuable dietetic intervention in selected patients with IBD.
Resource: Inflammatory Bowel Disease 2015 Feb 24 epub ahead of print.
Aziz A, Branchi F, Pearson K, Priest J, Sanders D.
Association between Coeliac Disease and Psoriasis: Italian Primary Care Multicentre Study
Coeliac disease is increasingly associated with extraintestinal manifestations, among these, several skin disorders have been reported, with dermatitis herpetiformis being the best recognised. Several studies investigating the possible association between CD and psoriasis have been published, however results have so far proven to be inconclusive and most of the data on the effect of a gluten free diet on psoriatic skin lesions are contained within case reports.
In this prospective multicentre study, the patient records from 19 Italian primary care practices were screened for confirmed cases of psoriasis, following this 219 patients with a confirmed diagnosis of psoriasis were enrolled in to the study (111 males, 51%; mean age 54 years), and 264 age and sex matched controls without psoriasis. The severity of psoriasis amongst subjects was assessed at base-line and follow up, in addition to gastrointestinal symptoms. Serum levels of anti-tTG were recorded for all subjects and if positive, CD diagnosis was confirmed by histology on biopsy. A total of 49% of the 218 enrolled patients with psoriasis reported one or more GI symptom, the most common being heartburn/ acid regurgitation (27%) and bloating (16%). Nine out of 218 patients with psoriasis showed positive serology for CD compared to only 1 out of the 264 controls (P=<0.05). Histology confirmed the CD diagnosis in all subjects with positivity to anti-tTG antibodies. All patients with psoriasis and CD adhered to a gluten free diet and completed the 6 month follow up. At 3 months, all 9 patients showed significant improvement in the severity of their psoriasis, this improvement was maintained at 6 months in all but 1 patient whose symptoms worsened.
This study shows a significant epidemiological association between CD and psoriasis in the Italian population. However, the small number of psoriatic patients with evidence of CD cannot be considered as definitive evidence of a causal link between the two disorders. The authors of this study hypothesise that the presence of shared genes (at-risk HLA haplotypes) often used to explain the greater prevalence of CD in several autoimmune disorders, might be present in subjects affected by psoriasis. In addition, CD-related malabsorption may affect psoriasis by causing a vitamin D deficiency status. It is well known that low levels of vitamin D pre-dispose to psoriasis, and that expose to sunlight and topical vitamin D preparations improve psoriatic lesions. Furthermore, an abnormal intestinal permeability common in untreated CD may increase the passage of different immune triggers. The fact that the majority of psoriatic subjects with positive CD serology showed complete villous atrophy is concordant with the hypotheses of vitamin D malabsorption and altered intestinal permeability to the passage of immune triggers.
Resource: Dermatology 2015 Feb 3.

Coeliac Disease in Women With Infertility - A Meta-Analysis
Although this study makes a case for screening women with infertility for coeliac disease. The authors acknowledge that their work alone cannot be used to prove a causal relationship between coeliac disease and infertility due to the limitation of the study design of case control studies, the small number of studies available overall and the small sample sizes used within these. A recent large population-based study of infertility and coeliac disease in UK women suggested that women with coeliac disease (both before and after diagnosis) do not have a greater chance of clinically recorded fertility problems than women without coeliac disease*. However, the design of this study has it's own limitations due to reliance on primary care records, the known mis-diagnosis of coeliac disease and delay in recording this within medical records.
Resource: Dhalwani N.N, West J et al (2014) Women with celiac disease present with fertility problems no more often than women in the general population. Gastroenterology 147: 1267-74

A Retrospective Application of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosis of Coeliac Disease in New Zealand Children.
Resource: International Journal of Coeliac Disease, Dec 2014 (vol 2; no. 4)

Is gluten the great etiopathogenic agent of disease in the XXI century?
Introduction: Gluten is a glycoprotein present in some cereals. The incidence of disorders related to gluten, including the EC, is increasing, even pathologies far from an etiology or treatment with GFD.
Aims: Review the scientific literature related to the ingestion of gluten and pathogenesis of different diseases.
Methods: A literature search in major scientific database.
Results: We obtained from the following diseases, gluten ataxia, multiple sclerosis, autism spectrum disorder, schizophrenia, attention deficit hyperactivity disorder, depressive disorders, headaches, irritable bowel syndrome, fibromyalgia, dermatitis herpetiformis and epilepsy, studies in which either a determination of gliadin was referred or a treatment, with/without gluten, was applied and evaluated.
Conclusion: The ingestion of gluten seems to be related to disease, when there is no EC, SGNC or wheat allergy. Suspicions about the benefit of GFD as a complementary treatment is borne in semi-clinical trials and cohorts, either as a causal factor in the pathogenesis, or improvement of symptoms.
Resource: Nutr Hosp. 2014;30(6):1203-1210
Ismael San Mauro Martín, Elena Garicano Vilar, Luis Collado Yurrutia y María José Ciudad Cabañas

Causes of death in people with coeliac disease in England compared with the general population: a competing risk analysis
Introduction:
Quantifying excess cause-specific mortality among people with coeliac disease (CD) compared with the general population accounting for competing risks will allow accurate information to be given on risk of death from specific causes.
Method:
We identified from the Clinical Practice Research Datalink all patients with CD linked to Office for National Statistics between 1998 and 2012. We selected controls by frequency matching from the registered general practice population within 10-year age bands. We calculated the adjusted cumulative incidence (including adjustment for competing risks) and excess cumulative incidence for different causes of death up to 10 years from diagnosis.
Results:
Of the 10 825 patients with CD, 773 died within the study period. The overall mortality rate among patients with CD was 128/10 000 person years compared with 153/10 000 in controls (HR=0.94 95% CI 0.84 to 1.01). We found no overall difference in the cumulative incidence of respiratory disease, digestive disease or cancer related death among cases and controls. The adjusted cumulative incidence of death from cardiovascular deaths was slightly lower compared with those without CD diagnosis (CD 0.32% vs controls 0.41%) with a corresponding excess cumulative incidence of −0.08% (95% CI −0.13 to −0.04). However, patients with CD had 0.15% excess risk (95% CI 0.03 to 0.27) of deaths from non-Hodgkin's lymphoma from the general population baseline risk.
Conclusions:
Overall, people with CD have no major excess risk of cancer, digestive disease or respiratory disease related or cardiovascular mortality compared with the general population. These findings should be reassuring to patients with CD and clinicians managing their care.
Resource: Abdul Sultan A, et al. Gut 2014;0:1–7
Alyshah Abdul Sultan, Colin J Crooks, Tim Card, Laila J Tata, Kate M Fleming, Joe West

Glycaemic index of some commercial gluten-free foods
Purpose
Gluten-free products present major challenges for the food industry in terms of organoleptic, technological and nutritional characteristics. The absence of gluten has been shown to affect starch digestibility, thus increasing the postprandial glycaemic response. However, in recent years, gluten-free technologies have been improved, thus possibly modifying this quality parameter. We investigated the glycaemic index (GI) of 10 commercial foods aiming to update the GI values of the most common gluten-free products consumed in Italy.
Methods
The in vivo GI was evaluated for six bakery products and four types of pasta. The postprandial glucose response was obtained in two groups with 10 healthy volunteers each.
Results
The overall GI values ranged from 37.5 for breakfast biscuits to 66.7 for puffed multigrain cake. Breads and pasta had GI values consistently lower than those previously reported in the literature.
Conclusion
The present study showed that several commercial GF products exhibited low and medium GI values, not confirming the previous observations on the high GI of GF. However, considering the multiple formulations and processes for preparation of these products, further studies are recommended.
Resource: Eur J Nutr. 2014 Oct 17.
Francesca Scazzina • Margherita Dall’Asta • Nicoletta Pellegrini • Furio Brighenti

Introduction of Gluten, HLA Status, and the Risk of Celiac Disease in Children
Background
The relationship between the risk of celiac disease and both the age at which gluten is introduced to a child’s diet and a child’s early dietary pattern is unclear.
Methods
We randomly assigned 832 newborns who had a first-degree relative with celiac disease to the introduction of dietary gluten at 6 months (group A) or 12 months (group B). The HLA genotype was determined at 15 months of age, and serologic screening for celiac disease was evaluated at 15, 24, and 36 months and at 5, 8, and 10 years. Patients with positive serologic findings underwent intestinal biopsies. The primary outcome was the prevalence of celiac disease autoimmunity and of overt celiac disease among the children at 5 years of age.
Results
Of the 707 participants who remained in the trial at 36 months, 553 had a standard-risk or high-risk HLA genotype and completed the study. At 2 years of age, significantly higher proportions of children in group A than in group B had celiac disease autoimmunity (16% vs. 7%, P=0.002) and overt celiac disease (12% vs. 5%, P=0.01). At 5 years of age, the between-group differences were no longer significant for autoimmunity (21% in group A and 20% in group B, P=0.59) or overt disease (16% and 16%, P=0.78 by the log-rank test). At 10 years, the risk of celiac disease autoimmunity was far higher among children with high-risk HLA than among those with standard-risk HLA (38% vs. 19%, P=0.001), as was the risk of overt celiac disease (26% vs. 16%, P=0.05). Other variables, including breast-feeding, were not associated with the development of celiac disease.
Conclusions
Neither the delayed introduction of gluten nor breast-feeding modified the risk of celiac disease among at-risk infants, although the later introduction of gluten was associated with a delayed onset of disease. A high-risk HLA genotype was an important predictor of disease.
Resource: N Engl J Med 2014; 371:1295-1303October 2, 2014DOI: 10.1056/NEJMoa1400697
Elena Lionetti, M.D., Stefania Castellaneta, M.D., Ruggiero Francavilla, M.D., Ph.D., Alfredo Pulvirenti, Ph.D., Elio Tonutti, M.D., Sergio Amarri, M.D., Maria Barbato, M.D., Cristiana Barbera, M.D., Graziano Barera, M.D., Antonella Bellantoni, M.D., Emanuela Castellano, M.D., Graziella Guariso, M.D., Maria Giovanna Limongelli, M.D., Salvatore Pellegrino, M.D., Carlo Polloni, M.D., Claudio Ughi, M.D., Giovanna Zuin, M.D., Alessio Fasano, M.D., and Carlo Catassi, M.D., M.P.H. for the SIGENP (Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition) Working Group on Weaning and CD Risk

Randomized Feeding Intervention in Infants at High Risk for Celiac Disease
A window of opportunity has been suggested for reducing the risk of celiac disease by introducing gluten to infants at 4 to 6 months of age.
Methods
We performed a multicenter, randomized, double-blind, placebo-controlled dietary-intervention study involving 944 children who were positive for HLA-DQ2 or HLA-DQ8 and had at least one first-degree relative with celiac disease. From 16 to 24 weeks of age, 475 participants received 100 mg of immunologically active gluten daily, and 469 received placebo. Anti–transglutaminase type 2 and antigliadin antibodies were periodically measured. The primary outcome was the frequency of biopsy-confirmed celiac disease at 3 years of age.
Results
Celiac disease was confirmed by means of biopsies in 77 children. To avoid underestimation of the frequency of celiac disease, 3 additional children who received a diagnosis of celiac disease according to the 2012 European Society for Pediatric Gastroenterology, Hepatology, and Nutrition diagnostic criteria (without having undergone biopsies) were included in the analyses (80 children; median age, 2.8 years; 59% were girls). The cumulative incidence of celiac disease among patients 3 years of age was 5.2% (95% confidence interval [CI], 3.6 to 6.8), with similar rates in the gluten group and the placebo group (5.9% [95% CI, 3.7 to 8.1] and 4.5% [95% CI, 2.5 to 6.5], respectively; hazard ratio in the gluten group, 1.23; 95% CI, 0.79 to 1.91). Rates of elevated levels of anti–transglutaminase type 2 and antigliadin antibodies were also similar in the two study groups (7.0% [95% CI, 4.7 to 9.4] in the gluten group and 5.7% [95% CI, 3.5 to 7.9] in the placebo group; hazard ratio, 1.14; 95% CI, 0.76 to 1.73). Breast-feeding, regardless of whether it was exclusive or whether it was ongoing during gluten introduction, did not significantly influence the development of celiac disease or the effect of the intervention.
Conclusions
As compared with placebo, the introduction of small quantities of gluten at 16 to 24 weeks of age did not reduce the risk of celiac disease by 3 years of age in this group of high-risk children.
Resource: N Engl J Med 2014; 371:1304-1315October 2, 2014DOI: 10.1056/NEJMoa1404172
Sabine L. Vriezinga, M.D., Renata Auricchio, M.D., Enzo Bravi, M.S., Gemma Castillejo, M.D., Anna Chmielewska, M.D., Ph.D., Paula Crespo Escobar, B.Sc., Sanja Kolacek, M.D., Ph.D., Sibylle Koletzko, M.D., Ph.D., Ilma R. Korponay-Szabo, M.D., Ph.D., Eckart Mummert, Ph.D., Isabel Polanco, M.D., Ph.D., Hein Putter, Ph.D., Carmen Ribes-Koninckx, M.D., Ph.D., Raanan Shamir, M.D., Ph.D., Hania Szajewska, M.D., Ph.D., Katharina Werkstetter, M.Sc., M.P.H., Luigi Greco, M.D., Ph.D., Judit Gyimesi, M.D., Corina Hartman, M.D., Caroline Hogen Esch, M.D., Ph.D., Erica Hopman, R.D., Ph.D., Anneli Ivarsson, M.D., Ph.D., Tunde Koltai, Ir., Frits Koning, Ph.D., Eva Martinez-Ojinaga, M.D., Chantal te Marvelde, B.Sc., Ana Pavic, M.D., Jihane Romanos, Ph.D., Els Stoopman, Vincenzo Villanacci, M.D., Ph.D., Cisca Wijmenga, Ph.D., Ricardo Troncone, M.D., Ph.D., and M. Luisa Mearin, M.D., Ph.D.

Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes
BACKGROUND:
In the past decade, a number of population-based studies have examined the prevalence of coeliac disease in individuals with type 1 diabetes but prevalences have differed considerably.
AIM:
To examine the prevalence of coeliac disease in individuals with type 1 diabetes.
METHODS:
A systematic review of English-language articles published in PubMed Medline between 2000 and May 2014. Search terms included 'celiac disease' or 'coeliac disease' and 'diabetes mellitus'. Studies were selected with at least 100 individuals with type 1 diabetes being screened for coeliac disease where the coeliac diagnosis was later confirmed through small intestinal biopsy. Data synthesis used random-effects inverse variance-weighted models, and metaregression was used to examine heterogeneity in subgroups.
RESULTS:
A pooled analysis, based on 26,605 patients with type 1 diabetes, found a prevalence of biopsy-confirmed coeliac disease of 6.0% (95% CI = 5.0-6.9%). Heterogeneity was large (I(2) = 93.2%). The prevalence was lower in adults with type 1 diabetes (2.7%), and in mixed populations with both children and adults with type 1 diabetes (4.7%) than in children (6.2%) with type 1 diabetes (P < 0.001). Additional subgroup analyses could not explain the large variation in coeliac disease prevalence between studies.
CONCLUSION:
More than one in twenty patients with type 1 diabetes have biopsy-verified coeliac disease. This prevalence is high enough to motivate screening for coeliac disease among patients with type 1 diabetes.
Resource: Aliment Pharmacol Ther. 2014 Nov;40(10):1123-32. doi: 10.1111/apt.12973. Epub 2014 Oct 1.
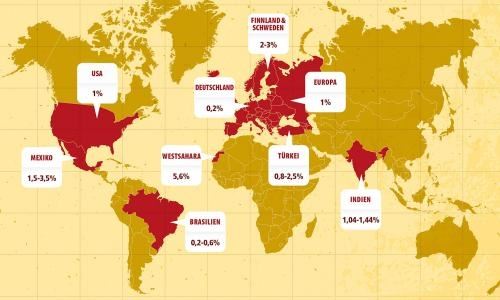
The New Epidemiology of Celiac Disease
The prevalence of celiac disease (CD) varies greatly, but several reports have shown that CD is increasing in frequency in different geographic areas. The increase in prevalence can be partially attributed to the improvement in diagnostic techniques and disease awareness; however the equally well documented rise in incidence in the last 30–40 years cannot be so easily explained. The new epidemiology of CD is now characterized by an increase of new cases in the historical CD areas (northern Europe and the United States) and more interestingly in a spread of the disease in new regions (Asian countries). A significant change in diet habits, particularly in gluten consumption as well as in infant feeding patterns are probably the main factors that can account for these new trends in CD epidemiology.
Resource: Journal of Pediatric Gastroenterology & Nutrition, July 2014 Volume 59

Celiachia: Dieci cose che ogni esperto nutrizionista e gastroenterologo dovrebbe sapere
Ecco qui un riepilogo di questi 10 punti
Ci sono 10 cose che ogni esperto nutrizionista e gastroenterologo dovrebbe sapere riguardo alla celiachia.
(1) L’analisi dell’immunoglobulina A nella transglutaminasi tissutale è la miglior prova sierologica per riconoscere la celiachia.
(2) La celiachia può essere diagnosticata tramite un’endoscopia e l’immersione in acqua migliora l’individuazione dei villi, ma nonostante tutto un’apparenza endoscopica normale non esclude una diagnosi.
(3) Si raccomanda di prelevare 4 biopsie dalla seconda parte del duodeno e 2 dal bulbo in posizione di ore 9 e 12 per massimizzare la sensibilità e quindi ottenere una conferma istologica della celiachia.
(4) È opportuno sottoporre a test sierologici parenti di primo grado, pazienti con diabete mellito di tipo 1, con sindrome di Down, sindrome di Turner o sindrome di Williams-Beuren, nonché tutti coloro che presentano osteoporosi prematura carenza di ferro, valori epatici anomali e altre manifestazioni di celiachia.
(5) Si consiglia di esaminare la presenza di HLA DQ2 o DQ8 in pazienti che già da tempo seguono una dieta senza glutine, in modo da evitare ulteriori valutazioni di celiachia in portatori non allelici.
(6) Il trattamento di base per la celiachia è una dieta senza glutine a vita, seguita da un dietista esperto. Tutti i pazienti che soffrono di celiachia sono tenuti a consultare un dietista specialista nell’alimentazione senza glutine. Sono attualmente disponibili delle linee guida per dietisti da consultare per il trattamento di pazienti affetti da celiachia. Se i pazienti intraprendono la ricerca riguardo alla dieta senza glutine individualmente, spesso nascono malintesi e la dieta viene ulteriormente limitata senza motivo. Altri aspetti pratici che i dietisti sono tenuti ad affrontare insieme ai loro pazienti comprendono il modo di evitare la contaminazione crociata (p. es. utilizzare tostapane e recipienti separati), consigli utili per il viaggio e per mangiare al ristorante, nonché dove trovare informazioni attendibili su Internet. Inoltre è necessario esaminare la salute generale di pazienti che seguono una dieta senza glutine, visto che l’obesità, il diabete ed altre malattie concomitanti sono sempre più frequenti.
(7) Nel caso di persone adulte neodiagnosticate di celiachia, si consiglia di esaminare carenze di vitamine e sali minerali (B12, folati, vitamina D, ferro, zinco e rame) e di prescrivere una densitometria ossea.
(8) È necessario che tutti i pazienti affetti da celiachia effettuino le visite follow-up per assicurare i risultati e l’aderenza alla dieta senza glutine.
(9) Per coloro che presentano sintomi persistenti o di ricaduta, è opportuno rivalutare la diagnosi originale, cercare eventuali fonti di glutine latenti e valutare sistematicamente malattie alternative o associate.
(10) Bisogna infine monitorare pazienti che presentano celiachia refrattaria per evitare evoluzioni maligne.
Resource: Clin Gastroenterol Hepatol. 2014 Jul 19. pii: S1542-3565(14)01053-2

Diets that differ in their FODMAP content alter the colonic luminal microenvironment.
OBJECTIVE:
A low FODMAP (Fermentable Oligosaccharides, Disaccharides, Monosaccharides And Polyols) diet reduces symptoms of IBS, but reduction of potential prebiotic and fermentative effects might adversely affect the colonic microenvironment. The effects of a low FODMAP diet with a typical Australian diet on biomarkers of colonic health were compared in a single-blinded, randomised, cross-over trial.
DESIGN:
Twenty-seven IBS and six healthy subjects were randomly allocated one of two 21-day provided diets, differing only in FODMAP content (mean (95% CI) low 3.05 (1.86 to 4.25) g/day vs Australian 23.7 (16.9 to 30.6) g/day), and then crossed over to the other diet with ≥21-day washout period. Faeces passed over a 5-day run-in on their habitual diet and from day 17 to day 21 of the interventional diets were pooled, and pH, short-chain fatty acid concentrations and bacterial abundance and diversity were assessed.
RESULTS:
Faecal indices were similar in IBS and healthy subjects during habitual diets. The low FODMAP diet was associated with higher faecal pH (7.37 (7.23 to 7.51) vs 7.16 (7.02 to 7.30); p=0.001), similar short-chain fatty acid concentrations, greater microbial diversity and reduced total bacterial abundance (9.63 (9.53 to 9.73) vs 9.83 (9.72 to 9.93) log10 copies/g; p<0.001) compared with the Australian diet. To indicate direction of change, in comparison with the habitual diet the low FODMAP diet reduced total bacterial abundance and the typical Australian diet increased relative abundance for butyrate-producing Clostridium cluster XIVa (median ratio 6.62; p<0.001) and mucus-associated Akkermansia muciniphila (19.3; p<0.001), and reduced Ruminococcus torques.
CONCLUSIONS:
Diets differing in FODMAP content have marked effects on gut microbiota composition. The implications of long-term reduction of intake of FODMAPs require elucidation.
Resource: Gut. 2014 Jul 12. pii: gutjnl-2014-307264. doi: 10.1136/gutjnl-2014-307264. [Epub ahead of print]
Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG.

Early nutrition: prevention of celiac disease?
Celiac disease (CD) is an autoimmune disorder caused by ingestion of gluten in genetically predisposed individuals. It is a chronic, multiorgan disease in which small-intestinal mucosal damage may lead to malabsorption of nutrients. In the last years, several studies suggested a protective role of breast-feeding and/or the timing and quantity of gluten introduction in the subsequent development of CD. Especially, prolonged breast feeding during the introduction of gluten-containing feeding has been associated with a reduced risk of developing CD in infancy. The focus of this review was to present the actual knowledge of the possible preventive influence of early nutrition on the risk to develop CD.
Resource: J Pediatr Gastroenterol Nutr. 2014 Jul;59 Suppl 1:S18-20
Schaart MW, Mearin ML

Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity
Background
Mild impairments of cognition or ‘Brain fog’ are often reported by patients with coeliac disease but the nature of these impairments has not been systematically investigated.
Aim
This longitudinal pilot study investigated relationships between cognitive function and mucosal healing in people with newly diagnosed coeliac disease commencing a gluten-free diet.
Methods
Eleven patients (8 females, 3 males), mean age 30 (range 22–39) years, were tested with a battery of cognitive tests at weeks 0, 12 and 52. Information processing efficacy, memory, visuospatial ability, motoric function and attention were tested. Small bowel biopsies were collected via routine gastroscopy at weeks 12 and 52 and were compared to baseline Marsh scores. Cognitive performance was compared to serum concentrations of tissue transglutaminase antibodies, biopsy outcomes and other biological markers.
Results
All patients had excellent adherence to the diet. Marsh scores improved significantly (P = 0.001, Friedman's test) and tissue transglutaminase antibody concentrations decreased from a mean of 58.4 at baseline to 16.8 U/mL at week 52 (P = 0.025). Four of the cognitive tests assessing verbal fluency, attention and motoric function showed significant improvement over the 12 months and strongly correlated with the Marsh scores and tissue transglutaminase antibody levels (r = 0.377–0.735; all P < 0.05). However, no meaningful patterns of correlations were found for nutritional or biochemical markers, or markers of intestinal permeability.
Conclusions
In newly diagnosed coeliac disease, cognitive performance improves with adherence to the gluten-free diet in parallel to mucosal healing. Suboptimal levels of cognition in untreated coeliac disease may affect the performance of everyday tasks.
Resource: Alimentary Pharmacology & Therapeutics Volume 40, Issue 2, pages 160–170, July 2014

Coeliac disease: The debate on coeliac disease screening - are we there yet?
Resource: Nature Reviews Gastroenterology & Hepatology Volume: 11, Pages: 457–458 201

Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology
A multidisciplinary panel of 18 physicians and 3 non-physicians from eight countries (Sweden, UK, Argentina, Australia, Italy, Finland, Norway and the USA) reviewed the literature on diagnosis and management of adult coeliac disease (CD).
This paper presents the recommendations of the British Society of Gastroenterology. Areas of controversies were explored through phone meetings and web surveys. Nine working groups examined the following areas of CD diagnosis and management: classification of CD; genetics and immunology; diagnostics; serology and endoscopy; follow-up; gluten-free diet; refractory CD and malignancies; quality of life; novel treatments; patient support; and screening for CD.
Resource: Gut doi:10.1136/gutjnl-2013-306578
Jonas F Ludvigsson, Julio C Bai, Federico Biagi, Timothy R Card, Carolina Ciacci, Paul J Ciclitira, Peter H R Green, Marios Hadjivassiliou, Anne Holdoway, David A van Heel, Katri Kaukinen, Daniel A Leffler, Jonathan N Leonard, Knut E A Lundin, Norma McGough, Mike Davidson, Joseph A Murray, Gillian L Swift, Marjorie M Walker, Fabiana Zingone, David S Sanders, Authors of the BSG Coeliac Disease Guidelines Development Group

Potential Celiac Children: 9-Year Follow-Up on a Gluten-Containing Diet
OBJECTIVES:
Potential celiac disease (CD) is defined by the presence of serum anti-tissue-transglutaminase (anti-TG2) antibodies and normal duodenal mucosa. The major clinical problem is the management of asymptomatic patients and how to predict the development of villous atrophy. This prospective longitudinal cohort study describes the natural history of potential CD up to 9 years and explores risk factors associated with the development of mucosal damage.
METHODS:
Two hundred and ten potential CD children were eligible for the study; 175/210 asymptomatic children were left on a gluten-containing diet. Antibodies and clinical symptoms were checked every 6 months, and a small bowel biopsy was taken every 2 years to evaluate histological, immunohistochemical, and anti-TG2 deposits. Patients were genotyped for HLA and a set of non-HLA CD-associated genes.
RESULTS:
Forty-three percent of patients showed persistently elevated anti-TG2 level, 20% became negative during follow-up, and 37% showed a fluctuant anti-TG2 course with transiently negative values. At 3 years of follow-up, 86% of cases remained potential; 73 and 67% still had normal duodenal architecture at 6 and 9 years, respectively. Male sex, slight mucosal inflammation at time 0, and a peculiar genetic profile delineate a cohort of individuals who were prone to develop mucosal damage during time.
CONCLUSIONS:
A sizeable proportion of asymptomatic potential celiac patients showed fluctuation or negativization of antibody production, and many of these, with persistently positive anti-TG2, did not develop mucosal damage after 9 years of follow-up. Celiac population is a multivariate aggregate of individuals with different genetic and phenotypic profiles. Caution is required before prescribing a gluten-free diet for life to asymptomatic individuals with potential CD.
Resource: The American Journal of Gastroenterology 109, 913-921 (June 2014)
Renata Auricchio, Antonella Tosco, Emanuela Piccolo, Martina Galatola, Valentina Izzo, Mariantonia Maglio, Francesco Paparo, Riccardo Troncone and Luigi Greco

Managing coeliac disease in patients with diabetes
The association between coeliac disease and type 1 diabetes has long been established. The combination of genetic susceptibility along with a potential role for gluten in the pathogenesis of autoimmunity makes defining gluten's role in type 1 diabetes extremely important. Evidence supporting the role of a gluten-free diet to improve complications associated with type 1 diabetes is not robust. However there is evidence to support improved growth, bone density and potentially the prevention of additional autoimmune diseases in patients with coeliac disease and type 1 diabetes. The gluten free diet is expensive and challenging to adhere to in people already on a modified diet. Early identification of those who have coeliac disease and would benefit from a gluten-free diet is of utmost importance to prevent complications associated with type 1 diabetes and coeliac disease.
Resource: Diabetes Obes Metab. 2014 May 9

Randomised clinical trial: Gluten may cause depression in subjects with non-coeliac gluten sensitivity – an exploratory clinical study
Background
Current evidence suggests that many patients with self-reported non-coeliac gluten sensitivity (NCGS) retain gastrointestinal symptoms on a gluten-free diet (GFD) but continue to restrict gluten as they report ‘feeling better’.
Aim
To investigate the notion that a major effect of gluten in those with NCGS is on mental state and not necessarily on gastrointestinal symptoms.
Methods
Twenty-two subjects (24–62 years, five male) with irritable bowel syndrome who had coeliac disease excluded but were symptomatically controlled on a GFD, undertook a double-blind cross-over study. Participants randomly received one of three dietary challenges for 3 days, followed by a minimum 3-day washout before crossing over to the next diet. Challenge gluten-free food was supplemented with gluten (16 g/day), whey (16 g/day) or not supplemented (placebo). End-points included mental state as assessed by the Spielberger State Trait Personality Inventory (STPI), cortisol secretion and gastrointestinal symptoms.
Results
Gluten ingestion was associated with higher overall STPI state depression scores compared to placebo [M = 2.03, 95% CI (0.55–3.51), P = 0.010] but not whey [M = 1.48, 95% CI (−0.14 to 3.10), P = 0.07]. No differences were found for other STPI state indices or for any STPI trait measures. No difference in cortisol secretion was identified between challenges. Gastrointestinal symptoms were induced similarly across all dietary challenges.
Conclusions
Short-term exposure to gluten specifically induced current feelings of depression with no effect on other indices or on emotional disposition. Gluten-specific induction of gastrointestinal symptoms was not identified. Such findings might explain why patients with non-coeliac gluten sensitivity feel better on a gluten-free diet despite the continuation of gastrointestinal symptoms.
Resource: Alimentary Pharmacology & Therapeutics, Volume 39, Issue 10, pages 1104–1112, May 2014
S. L. Peters, J. R. Biesiekierski, G. W. Yelland, J. G. Muir1 and P. R. Gibson

Celiac Disease or Non-Celiac Gluten Sensitivity? An Approach to Clinical Differential Diagnosis
OBJECTIVES: Differentiating between celiac disease (CD) and non-celiac gluten sensitivity (NCGS) is important for appropriate management but is often challenging.
METHODS: We retrospectively reviewed records from 238 patients who presented for the evaluation of symptoms responsive to gluten restriction without prior diagnosis or exclusion of CD. Demographics, presenting symptoms, serologic, genetic, and histologic data, nutrient deficiencies, personal history of autoimmune diseases, and family history of CD were recorded. NCGS was defined as symptoms responsive to a gluten-free diet (GFD) in the setting of negative celiac serology and duodenal biopsies while on a gluten-containing diet or negative human leukocyte antigen (HLA) DQ2/DQ8 testing.
RESULTS: Of the 238 study subjects, 101 had CD, 125 had NCGS, 9 had non-celiac enteropathy, and 3 had indeterminate diagnosis. CD subjects presented with symptoms of malabsorption 67.3% of the time compared with 24.8% of the NCGS subjects (P<0.0001). In addition, CD subjects were significantly more likely to have a family history of CD (P=0.004), personal history of autoimmune diseases (P=0.002), or nutrient deficiencies (P<0.0001). The positive likelihood ratio for diagnosis of CD of a >2× upper limit of normal IgA trans-glutaminase antibody (tTG) or IgA/IgG deaminated gliadan peptide antibody (DGP) with clinical response to GFD was 130 (confidence interval (CI): 18.5–918.3). The positive likelihood ratio of the combination of gluten-responsive symptoms and negative IgA tTG or IgA/IgG DGP on a regular diet for NCGS was 9.6 (CI: 5.5–16.9). When individuals with negative IgA tTG or IgA/IgG DGP also lacked symptoms of malabsorption (weight loss, diarrhea, and nutrient deficiencies) and CD risk factors (personal history of autoimmune diseases and family history of CD), the positive likelihood ratio for NCGS increased to 80.9.
CONCLUSIONS: On the basis of our findings, we have developed a diagnostic algorithm to differentiate CD from NCGS. Subjects with negative celiac serologies (IgA tTG or IgA/IgG DGP) on a regular diet are unlikely to have CD. Those with negative serology who also lack clinical evidence of malabsorption and CD risk factors are highly likely to have NCGS and may not require further testing. Those with equivocal serology should undergo HLA typing to determine the need for biopsy.
Resource: The American Journal of Gastroenterology 109, 741-746 (May 2014)
Toufic A Kabbani, Rohini R Vanga, Daniel A Leffler, Javier Villafuerte-Galvez, Kumar Pallav, Joshua Hansen, Rupa Mukherjee, Melinda Dennis and Ciaran P Kelly
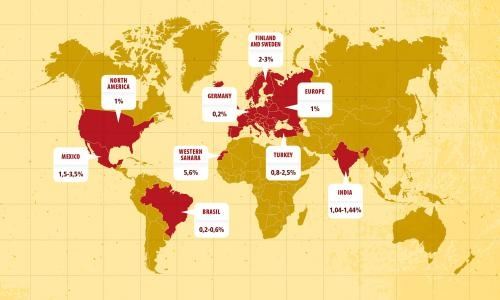
Incidence and Prevalence of Celiac Disease and Dermatitis Herpetiformis in the UK Over Two Decades: Population-Based Study
OBJECTIVES:
Few studies have quantified the incidence and prevalence of celiac disease (CD) and dermatitis herpetiformis (DH) nationally and regionally by time and age groups. Understanding this epidemiology is crucial for hypothesizing about causes and quantifying the burden of disease.
METHODS:
Patients with CD or DH were identified in the Clinical Practice Research Datalink between 1990 and 2011. Incidence rates and prevalence were calculated by age, sex, year, and region of residence. Incidence rate ratios (IRR) adjusted for age, sex, and region were calculated with Poisson regression.
RESULTS:
A total of 9,087 incident cases of CD and 809 incident cases of DH were identified. Between 1990 and 2011, the incidence rate of CD increased from 5.2 per 100,000 (95% confidence interval (CI), 3.8–6.8) to 19.1 per 100,000 person-years (95% CI, 17.8–20.5; IRR, 3.6; 95% CI, 2.7–4.8). The incidence of DH decreased over the same time period from 1.8 per 100,000 to 0.8 per 100,000 person-years (average annual IRR, 0.96; 95% CI, 0.94–0.97). The absolute incidence of CD per 100,000 person-years ranged from 22.3 in Northern Ireland to 10 in London. There were large regional variations in prevalence for CD but not DH.
CONCLUSIONS:
We found a fourfold increase in the incidence of CD in the United Kingdom over 22 years, with large regional variations in prevalence. This contrasted with a 4% annual decrease in the incidence of DH, with minimal regional variations in prevalence. These contrasts could reflect differences in diagnosis between CD (serological diagnosis and case finding) and DH (symptomatic presentation) or the possibility that diagnosing and treating CD prevents the development of DH.
Resource: Am J Gastroenterol. May 2014

Characterization of Adults With a Self-Diagnosis of Nonceliac Gluten Sensitivity
Background: Nonceliac gluten sensitivity (NCGS), occurring in patients without celiac disease yet whose gastrointestinal symptoms improve on a gluten-free diet (GFD), is largely a self-reported diagnosis and would appear to be very common. The aims of this study were to characterize patients who believe they have NCGS.
Materials and Methods: Advertising was directed toward adults who believed they had NCGS and were willing to participate in a clinical trial. Respondents were asked to complete a questionnaire about symptoms, diet, and celiac investigation.
Results: Of 248 respondents, 147 completed the survey. Mean age was 43.5 years, and 130 were women. Seventy-two percent did not meet the description of NCGS due to inadequate exclusion of celiac disease (62%), uncontrolled symptoms despite gluten restriction (24%), and not following a GFD (27%), alone or in combination. The GFD was self-initiated in 44% of respondents; in other respondents it was prescribed by alternative health professionals (21%), dietitians (19%), and general practitioners (16%). No celiac investigations had been performed in 15% of respondents. Of 75 respondents who had duodenal biopsies, 29% had no or inadequate gluten intake at the time of endoscopy. Inadequate celiac investigation was common if the GFD was initiated by self (69%), alternative health professionals (70%), general practitioners (46%), or dietitians (43%). In 40 respondents who fulfilled the criteria for NCGS, their knowledge of and adherence to the GFD were excellent, and 65% identified other food intolerances.
Conclusions: Just over 1 in 4 respondents self-reporting as NCGS fulfill criteria for its diagnosis. Initiation of a GFD without adequate exclusion of celiac disease is common. In 1 of 4 respondents, symptoms are poorly controlled despite gluten avoidance.
Resource: Nutr Clin Pract April 16, 2014
Jessica R. Biesiekierski, PhD, RN, Evan D. Newnham , MD, FRACP, Susan J. Shepherd, PhD, APD, Jane G. Muir, PhD, APD, Peter R. Gibson, MD, FRACP

Serological Assessment for Celiac Disease in IgA Deficient Adults
Purpose: Selective immunoglobulin A deficiency is the most common primary immunodeficiency disorder that is strongly overrepresented among patients with celiac disease (CD). IgG antibodies against tissue transglutaminase (tTG) and deamidated gliadin peptides (DGP) serve as serological markers for CD in IgA deficient individuals, although the diagnostic value remains uncertain. The aim of this study was to investigate the prevalence of these markers in a large cohort of IgA deficient adults with confirmed or suspected CD and relate the findings to gluten free diet.
Methods: Sera from 488,156 individuals were screened for CD in seven Swedish clinical immunology laboratories between 1998 and 2012. In total, 356 out of 1,414 identified IgA deficient adults agreed to participate in this study and were resampled. Forty-seven IgA deficient blood donors served as controls. Analyses of IgG antibodies against tTG and DGP as well as HLA typing were performed and a questionnaire was used to investigate adherence to gluten free diet. Available biopsy results were collected.
Results: Out of the 356 IgA deficient resampled adults, 67 (18.8%) were positive for IgG anti-tTG and 79 (22.2%) for IgG anti-DGP, 54 had biopsy confirmed CD. Among the 47 IgA deficient blood donors, 4 (9%) were positive for IgG anti-tTG and 8 (17%) for anti-DGP. Four were diagnosed with biopsy verified CD, however, 2 of the patients were negative for all markers. Sixty-eight of 69 individuals with positive IgG anti-tTG were HLA-DQ2/DQ8 positive whereas 7 (18.9%) of the 37 individuals positive for IgG anti-DGP alone were not.
Conclusions: IgG anti-tTG seems to be a more reliable marker for CD in IgA deficient adults whereas the diagnostic specificity of anti-DGP appears to be lower. High levels of IgG antibodies against tTG and DGP were frequently found in IgA deficient adults despite adhering to gluten free diet.
Resource: PLOS ONE, 9 April 2014, Vol 9, Issue 4 (www.plosone.org)

Psyllium as a substitute for gluten in pastas
The aim of the present study was to evaluate the effect of replacing gluten in favor of psyllium in pasta’s characteristics. This study takes an exploratory and quantitative approach and was sub-divided into four steps: selection and development of recipes and chemical and sensorial analysis. Modified samples of the pasta presented 100.0% of acceptance for individuals with celiac disease and up to 94.0% for individuals without celiac disease. The most affected characteristics were odor and texture. In terms of chemical composition, reduction of energy value was 26.5% and of proportional fat was 85.4% before being cooked. Substituting wheat flour for a mixture of gluten-free flours with psyllium did not alter preference or acceptability of modified products in relation to standardized ones and amplified feeding options for celiac disease patients. Thus, there were no damages in sensorial characteristics of these products.
Resource: Journal of Culinary Science & Technology Volume 12, Issue 2, 2014
Renata Puppin Zandonadi, Raquel Braz Assunção Botelho and Wilma Maria Coelho Araújo

Coeliac Patients Are Undiagnosed at Routine Upper Endoscopy
Background and Aims
Two out of three patients with Coeliac Disease (CD) in Australia are undiagnosed. This prospective clinical audit aimed to determine how many CD patients would be undiagnosed if duodenal biopsy had only been performed if the mucosa looked abnormal or the patient presented with typical CD symptoms.
Methods
All eligible patients presenting for upper gastrointestinal endoscopy (OGD) in a regional center from 2004–2009 underwent prospective analysis of presenting symptoms and duodenal biopsy. Clinical presentations were defined as either Major (diarrhea, weight loss, iron deficiency, CD family history or positive celiac antibodies- Ab) or Minor Clinical Indicators (CI) to duodenal biopsy (atypical symptoms). Newly diagnosed CD patients had follow up celiac antibody testing.
Results
Thirty-five (1.4%) new cases of CD were identified in the 2,559 patients biopsied at upper endoscopy. Almost a quarter (23%) of cases presented with atypical symptoms. There was an inverse relationship between presentation with Major CI’s and increasing age (<16, 16–59 and >60: 100%, 81% and 50% respectively, p = 0.03); 28% of newly diagnosed CD patients were aged over 60 years. Endoscopic appearance was a useful diagnostic tool in only 51% (18/35) of CD patients. Coeliac antibodies were positive in 34/35 CD patients (sensitivity 97%).
Conclusions
Almost one quarter of new cases of CD presented with atypical symptoms and half of the new cases had unremarkable duodenal mucosa. At least 10% of new cases of celiac disease are likely to be undiagnosed at routine upper endoscopy, particularly patients over 60 years who more commonly present atypically. All new CD patients could be identified in this study by performing pre-operative celiac antibody testing on all patients presenting for OGD and proceeding to biopsy only positive antibody patients and those presenting with either Major CI or abnormal duodenal mucosa for an estimated cost of AUS$4,629 and AUS$3,710 respectively.
Resource: PLOS ONE, March 2014, Vol 9, Issue 3 (www.plosone.org)

Coeliac disease
Adult coeliac disease is a common autoimmune condition with an estimated prevalence of 1%. Test for coeliac disease in patients with unexplained anaemia, weight loss, diarrhoea, or gastrointestinal symptoms, particularly irritable bowel syndrome, and in first degree relatives of index cases. Confirm the diagnosis with duodenal biopsy in all adult patients. Treatment with a lifelong strict gluten-free diet is currently the only treatment of known effectiveness. Patients should have access to an expert dietitian for advice on a gluten-free diet and for assessment of adherence if symptoms persist on institution of the diet. Regular follow-up is necessary to assess adherence and micronutrient deficiency.
Coeliac disease is a common autoimmune condition characterised by a heightened immunological response to ingested gluten, with estimated prevalence rates in adults of 0.2-1% in the United States and Europe. Contemporary studies suggest that the prevalence of this disease is increasing.3 4 5 Meta-analyses have shown that for every patient identified as having coeliac disease seven to eight remain undiagnosed. Here, we will summarise recent evidence on how the investigation and diagnosis of coeliac disease can be improved and also provide an evidence based approach to managing patients with newly diagnosed coeliac disease and those who do not respond to a gluten-free diet as expected. Evidence is taken from meta-analyses, systematic reviews, and randomised controlled trials where possible.
Sources and selection criteria
We searched Medline and the Cochrane Database of Systematic Reviews with the search terms “coeliac disease” or “celiac disease”. Studies included those in adult and paediatric populations but preference was given to adult studies in the past five years. We focused on meta-analyses and systematic reviews where possible.
Who gets coeliac disease?
In the past coeliac disease was considered to be a disease that affects white populations only, but it is now clear that coeliac disease is a global problem. Clinicians in …
Resource: BMJ 2014; (Published 3 March 2014)
Peter D Mooney, clinical research fellow gastroenterology, Marios Hadjivassiliou, professor of neurology and NHS consultant, David S Sanders, professor of gastroenterology and NHS consultant

Persistent mucosal damage and risk of fracture in celiac disease.
CONTEXT:
Celiac disease (CD) is associated with an increased fracture risk, an increase that persists after diagnosis. A significant proportion of patients with CD have persistent villous atrophy (VA) on follow-up biopsy.
OBJECTIVE:
The objective of the study was to determine whether persistent VA impacts long-term fracture risk.
DESIGN:
This was a cohort study.
SETTING AND PATIENTS:
We identified all patients in Sweden with histological evidence of CD who underwent a follow-up biopsy and compared patients with persistent VA with those with mucosal healing.
MAIN OUTCOME MEASURES:
The following were measured: 1) any fracture; 2) likely osteoporotic fracture (defined as fractures of the hip, distal forearm, thoracic and lumbar spine, or proximal humerus); and 3) hip fracture.
RESULTS:
Of 7146 patients, VA was present on follow-up biopsy in 43%. There was no significant association between persistent VA and overall fractures [hazard ratio (HR) of persistent VA compared with those with healing 0.93, 95% confidence interval (CI) 0.82-1.06] or with likely osteoporotic fractures (HR 1.11, 95% CI 0.84-1.46). Persistent VA was associated with an increased risk of hip fracture (HR 1.67, 95% CI 1.05-2.66). Hip fracture risk increased, depending on the degree of VA (HR for partial VA compared with those with healing 1.70, 95% CI 0.82-3.49, HR for subtotal/total VA compared with those with healing 2.16, 95% CI 1.06-4.41).
CONCLUSIONS:
Persistent VA on follow-up biopsy is predictive of hip fracture risk. The association between persistent VA and hip fractures, but not fractures overall, implies that thinner sc tissue and fall or trauma may be mechanisms by which persistent VA confers an increased fracture risk.
Resource: J Clin Endocrinol Metab. 2014 Feb

Glutenase ALV003 Attenuates Gluten-Induced Mucosal Injury in Patients With Celiac Disease
BACKGROUND & AIMS
Gluten ingestion leads to small intestinal mucosal injury in patients with celiac disease, necessitating strict life-long exclusion of dietary gluten. Despite adherence to a glutenfree diet, many patients remain symptomatic and still have small intestinal inflammation. In this case, nondietary therapies are needed. We investigated the ability of ALV003, a mixture of 2 recombinant gluten-specific proteases given orally, to protect patients with celiac disease from gluten-inducedmucosal injury in a phase 2 trial.
METHODS
We established the optimal daily dose of gluten to be used in a 6-week challenge study. Then, in the intervention study, adults with biopsy-proven celiac disease were randomly assigned to groups given ALV003 (n ¼ 20) or placebo (n ¼ 21) together with the daily gluten challenge. Duodenal biopsies were collected at baseline and after gluten challenge. The ratio of villus height to crypt depth and densities of intraepithelial lymphocytes were the primary end points.
RESULTS
A daily dose of 2g gluten was selected for the intervention study. Sixteen patients given ALV003 and 18 given placebo were eligible for efficacy evaluation. Biopsies from subjects in the placebo group showed evidence of mucosal injury after gluten challenge (mean villus height to crypt depth ratio changed from 2.8 before challenge to 2.0 afterward; P ¼ .0007; density of CD3þ intraepithelial lymphocytes changed from 61 to 91 cells/mm after challenge; P ¼ .0003). However, no significant mucosal deterioration was observed in biopsies from the ALV003 group. Between groups, morphologic changes and CD3þ intraepithelial lymphocyte counts differed significantly from baseline to week 6 (P ¼ .0133 and P ¼ .0123, respectively). There were no statistically significant differences in symptoms between groups.
CONCLUSIONS
Based on a phase 2 trial, the glutenase ALV003 appears to attenuate gluten-induced small intestinal mucosal injury in patients with celiac disease in the context of an everyday gluten-free diet containing daily up to 2 g gluten.
Resource: Gastroenterology 2014;146:1649–1658
Marja-Leena Lähdeaho, Katri Kaukinen, Kaija Laurila, Pekka Vuotikka, Olli-Pekka Koivurova, Tiina Kärjä-Lahdensuu, Annette Marcantonio, Daniel C. Adelman, and Markku Mäki

Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity
BACKGROUND:
Non-celiac gluten sensitivity is a syndrome characterized by gastrointestinal and extra-intestinal symptoms occurring in a few hours/days after gluten and/or other wheat protein ingestion and rapidly improving after exclusion of potential dietary triggers. There are no established laboratory markers for non-celiac gluten sensitivity, although a high prevalence of first generation anti-gliadin antibodies of IgG class has been reported in this condition. This study was designed to characterize the effect of the gluten-free diet on anti-gliadin antibodies of IgG class in patients with non-celiac gluten sensitivity.
METHODS:
Anti-gliadin antibodies of both IgG and IgA classes were assayed by ELISA in 44 non-celiac gluten sensitivity and 40 celiac disease patients after 6 months of gluten-free diet.
RESULTS:
The majority of non-celiac gluten sensitivity patients (93.2%) showed the disappearance of anti-gliadin antibodies of IgG class after 6 months of gluten-free diet; in contrast, 16/40 (40%) of celiac patients displayed the persistence of these antibodies after gluten withdrawal. In non-celiac gluten sensitivity patients anti-gliadin antibodies IgG persistence after gluten withdrawal was significantly correlated with the low compliance to gluten-free diet and a mild clinical response.
CONCLUSIONS:
Anti-gliadin antibodies of the IgG class disappear in patients with non-celiac gluten sensitivity reflecting a strict compliance to the gluten-free diet and a good clinical response to gluten withdrawal.
Resource: BMC Gastroenterol. 2014 Feb 13
Caio G, Volta U, Tovoli F, De Giorgio R

Follow-up of pediatric celiac disease: value of antibodies in predicting mucosal healing, a prospective cohort study
Background: In diagnosing celiac disease (CD), serological tests are highly valuable. However, their role in following up children with CD after prescription of a gluten-free diet is unclear. This study aimed to compare the performance of antibody tests in predicting small-intestinal mucosal status in diagnosis vs. follow-up of pediatric CD.
Methods: We conducted a prospective cohort study at a tertiary-care center. 148 children underwent esophohagogastroduodenoscopy with biopsies either for symptoms ± positive CD antibodies (group A; n = 95) or following up CD diagnosed ≥ 1 year before study enrollment (group B; n = 53). Using biopsy (Marsh ≥ 2) as the criterion standard, areas under ROC curves (AUCs) and likelihood-ratios were calculated to estimate the performance of antibody tests against tissue transglutaminase (TG2), deamidated gliadin peptide (DGP) and endomysium (EMA).
Results: AUCs were higher when tests were used for CD diagnosis vs. follow-up: 1 vs. 0.86 (P = 0.100) for TG2-IgA, 0.85 vs. 0.74 (P = 0.421) for TG2-IgG, 0.97 vs. 0.61 (P = 0.004) for DPG-IgA, and 0.99 vs. 0.88 (P = 0.053) for DPG-IgG, respectively. Empirical power was 85% for the DPG-IgA comparison, and on average 33% (range 13–43) for the non-significant comparisons. Among group B children, 88.7% showed mucosal healing (median 2.2 years after primary diagnosis). Only the negative likelihood-ratio of EMA was low enough (0.097) to effectively rule out persistent mucosal injury. However, out of 12 EMA-positive children with mucosal healing, 9 subsequently turned EMA-negative.
Conclusions: Among the CD antibodies examined, negative EMA most reliably predict mucosal healing. In general, however, antibody tests, especially DPG-IgA, are of limited value in predicting the mucosal status in the early years post-diagnosis but may be sufficient after a longer period of time.
Resource: BMC Gastroenterology 2014, 14:28

Early feeding and risk of celiac disease in a prospective birth cohort
OBJECTIVES:
Timing of gluten introduction has been associated with the risk of celiac disease (CD) in children, but the optimal time window is unknown. We aimed to study the effect of age of gluten introduction on the risk of CD, adjusting for continued breastfeeding.
METHODS:
In The Norwegian Mother and Child Cohort Study, a prospective birth cohort including 107,000 children, CD was identified by questionnaires and by linkage to the Norwegian Patient Register. Gluten introduction was reported monthly from 0 to 6 months of age, and breastfeeding from 0 to 18 months.
RESULTS:
After exclusion of cases with insufficient information, 324 children with CD in a cohort of 82,167 were used in the analyses. Gluten was introduced before or at 4 months in 8.0%, 5 to 6 months in 45.3%, and after 6 months in 46.6%, whereas continued breastfeeding was stable at ≈ 78% at 6 months age. CD was diagnosed in 3.68/1000 of the infants with gluten introduction at 5 to 6 months compared with 4.15/1000 with late and 4.24/1000 with early gluten introduction. After adjustment for the child's age and gender, breastfeeding, and maternal CD, delayed gluten introduction was associated with an increased risk of CD (adjusted odds ratio, 1.27 [95% confidence interval, 1.01-1.65], P = .045). Breastfeeding >12 months was also associated with increased risk (adjusted odds ratio, 1.49 [95% confidence interval, 1.01-2.21], P = .046).
CONCLUSIONS:
We found an increased risk of CD in children introduced to gluten after 6 months and a higher risk in children breastfed after 12 months age.
Resource: Pediatrics. 2013 Nov;132(5):e1202-9. doi: 10.1542/peds.2013-1752. Epub 2013 Oct 7.
Størdal K, White RA, Eggesbø M.
A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome
Background & Aims: A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) often is used to manage functional gastrointestinal symptoms in patients with irritable bowel syndrome (IBS), yet there is limited evidence of its efficacy, compared with a normal Western diet. We investigated the effects of a diet low in FODMAPs compared with an Australian diet, in a randomized, controlled, single-blind, cross-over trial of patients with IBS.
Methods: In a study of 30 patients with IBS and 8 healthy individuals (controls, matched for demographics and diet), we collected dietary data from subjects for 1 habitual week. Participants then randomly were assigned to groups that received 21 days of either a diet low in FODMAPs or a typical Australian diet, followed by a washout period of at least 21 days, before crossing over to the alternate diet. Daily symptoms were rated using a 0- to 100-mm visual analogue scale. Almost all food was provided during the interventional diet periods, with a goal of less than 0.5 g intake of FODMAPs per meal for the low-FODMAP diet. All stools were collected from days 17–21 and assessed for frequency, weight, water content, and King's Stool Chart rating.
Results: Subjects with IBS had lower overall gastrointestinal symptom scores (22.8; 95% confidence interval, 16.7–28.8 mm) while on a diet low in FODMAPs, compared with the Australian diet (44.9; 95% confidence interval, 36.6–53.1 mm; P < .001) and the subjects' habitual diet. Bloating, pain, and passage of wind also were reduced while IBS patients were on the low-FODMAP diet. Symptoms were minimal and unaltered by either diet among controls. Patients of all IBS subtypes had greater satisfaction with stool consistency while on the low-FODMAP diet, but diarrhea-predominant IBS was the only subtype with altered fecal frequency and King's Stool Chart scores.
Conclusions: In a controlled, cross-over study of patients with IBS, a diet low in FODMAPs effectively reduced functional gastrointestinal symptoms. This high-quality evidence supports its use as a first-line therapy.
Resource: Gastroenterology Volume 146, Issue 1 , Pages 67-75.e5, January 2014
Emma P. Halmos, Victoria A. Power, Susan J. Shepherd, Peter R. Gibson, Jane G. Muir

Celiac disease: diagnosis and management.
Celiac disease is an autoimmune disorder of the gastrointestinal tract. It is triggered by exposure to dietary gluten in genetically susceptible individuals. Gluten is a storage protein in wheat, rye, and barley, which are staples in many American diets. Celiac disease is characterized by chronic inflammation of the small intestinal mucosa, which leads to atrophy of the small intestinal villi and subsequent malabsorption. The condition may develop at any age. Intestinal manifestations include diarrhea and weight loss. Common extraintestinal manifestations include iron deficiency anemia, decreased bone mineral density, and neuropathy. Most cases of celiac disease are diagnosed in persons with extraintestinal manifestations. The presence of dermatitis herpetiformis is pathognomonic for celiac disease. Diagnosis is supported by a positive tissue transglutaminase serologic test but, in general, should be confirmed by a small bowel biopsy showing the characteristic histology associated with celiac disease. The presence of human leukocyte antigen alleles DQ2, DQ8, or both is essential for the development of celiac disease, and can be a useful genetic test in select instances. Treatment of celiac disease is a gluten-free diet. Dietary education should focus on identifying hidden sources of gluten, planning balanced meals, reading labels, food shopping, dining out, and dining during travel. About 5% of patients with celiac disease are refractory to a gluten-free diet. These patients should be referred to a gastroenterologist for reconsideration of the diagnosis or for aggressive treatment of refractory celiac disease, which may involve corticosteroids and immunomodulators.
Resource: Am Fam Physician. 2014 Jan 15;89(2):99-105.

Usefulness of Symptoms to Screen for Celiac Disease
OBJECTIVE:
To describe the frequency of symptoms and associated conditions among screening-detected celiac disease (CD) cases and non-CD children and to evaluate questionnaire-based case-finding targeting the general population.
METHODS:
In a population-based CD screening of 12-year-olds, children and their parents completed questionnaires on CD-associated symptoms and conditions before knowledge of CD status. Questionnaire data for those who had their CD detected in the screening (n = 153) were compared with those of children with normal levels of CD markers (n = 7016). Hypothetical case-finding strategies were also evaluated. Questionnaires were returned by 7054 (98%) of the children and by 6294 (88%) of their parents.
RESULTS:
Symptoms were as common among screening-detected CD cases as among non-CD children. The frequency of children with screening-detected CD was similar when comparing the groups with and without any CD-related symptoms (2.1% vs 2.1%; P = .930) or CD-associated conditions (3.6% vs 2.1%; P = .07). Case-finding by asking for CD-associated symptoms and/or conditions would have identified 52 cases (38% of all cases) at a cost of analyzing blood samples for 2282 children (37%) in the study population.
CONCLUSIONS:
The current recommended guidelines for finding undiagnosed CD cases, so-called active case-finding, fail to identify the majority of previously undiagnosed cases if applied in the general population of Swedish 12-year-olds. Our results warrant further studies on the effectiveness of CD case-finding in the pediatric population, both at the clinical and population-based levels.
Resource: Pediatrics. 2014 Feb;133(2):211-8. doi: 10.1542/peds.2012-3765. Epub 2014 Jan 13

Prospective Study of Clinical and Histological Safety of Pure and Uncontaminated Canadian Oats in the Management of Celiac Disease
Background:
Pure oats are safe for most patients with celiac disease, but concerns regarding contamination by other grains limit their consumption. The Canadian Celiac Association recently released guidelines governing the production of pure oats. The objective was to test the safety of a product manufactured under these guidelines.
Methods:
Fifteen adults with established, biopsy-confirmed celiac disease of =1 year duration were challenged with 350 g/wk of pure oats for 12 weeks. Symptom scores, weight, hemoglobin, ferritin, albumin, and tissue transglutaminase (tTG) were assessed at weeks 0, 6, and 12. Duodenal biopsies were obtained before and after oat challenge and assessed based on the modified Marsh–Oberhuber score. Compliance with a gluten-free diet was monitored with random food diaries.
Results:
Fifteen patients completed the study and were analyzed in intention-to-treat and per-protocol analyses. There were no significant changes in symptom scores, weight, hemoglobin, ferritin, or albumin during oat consumption. The tTG remained negative in all patients, and the histology scores did not significantly change during oat challenge. The only relapse occurred in a patient who became noncompliant with her gluten-free diet. Conclusion: The findings support the safety of pure, uncontaminated oats manufactured under Canadian Celiac Association guidelines for patients with celiac disease.
Resource: JPEN J Parenter Enteral Nutr July 2011 vol. 35 no. 4 459-464
Michael Sai Lai Sey, MD, Jeremy Parfitt, MD, FRCPC, Jamie Gregor, MD, FRCPC
Dieta vegetariana e celiachia

Diagnosi di sensibilità al glutine non celiaca (SGNC) in pazienti con sintomi funzionali gastrointestinali: risultati di uno studio multicentrico, randomizzato, in doppio cieco contro placebo con provocazione con glutine.

L’impiego dell’avena nella dieta senza glutine

Da oggi a tavola c’è … una nuova opportunità: l’AVENA

Revisione sistematica con meta-analisi: nutrizione nella prima infanzia e malattia celiaca – aggiornata al 2015.

Non-celiac gluten sensitivity has narrowed the spectrum of irritable bowel syndrome: A double-blind randomized placebo-controlled trial

Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria

Small Amounts of Gluten in Subjects With Suspected Nonceliac Gluten Sensitivity: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial.

Clinical effectiveness and economic costs of group versus one-to-one education for short-chain fermentable carbohydrate restriction (low FODMAP diet) in the management of irritable bowel syndrome.

Systematic review: non coeliac gluten sensitivity

A Study Evaluating the Bidirectional Relationship Between Inflammatory Bowel Disease and Self-reported Non-coeliac Gluten Sensitivity.

Association between Coeliac Disease and Psoriasis: Italian Primary Care Multicentre Study

Coeliac Disease in Women With Infertility - A Meta-Analysis

A Retrospective Application of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosis of Coeliac Disease in New Zealand Children.

Is gluten the great etiopathogenic agent of disease in the XXI century?

Causes of death in people with coeliac disease in England compared with the general population: a competing risk analysis

Glycaemic index of some commercial gluten-free foods

Introduction of Gluten, HLA Status, and the Risk of Celiac Disease in Children

Randomized Feeding Intervention in Infants at High Risk for Celiac Disease

Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes

The New Epidemiology of Celiac Disease

Celiachia: Dieci cose che ogni esperto nutrizionista e gastroenterologo dovrebbe sapere

Diets that differ in their FODMAP content alter the colonic luminal microenvironment.

Early nutrition: prevention of celiac disease?

Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity

Coeliac disease: The debate on coeliac disease screening - are we there yet?

Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology

Potential Celiac Children: 9-Year Follow-Up on a Gluten-Containing Diet

Managing coeliac disease in patients with diabetes

Randomised clinical trial: Gluten may cause depression in subjects with non-coeliac gluten sensitivity – an exploratory clinical study

Celiac Disease or Non-Celiac Gluten Sensitivity? An Approach to Clinical Differential Diagnosis

Incidence and Prevalence of Celiac Disease and Dermatitis Herpetiformis in the UK Over Two Decades: Population-Based Study

Characterization of Adults With a Self-Diagnosis of Nonceliac Gluten Sensitivity

Serological Assessment for Celiac Disease in IgA Deficient Adults

Psyllium as a substitute for gluten in pastas

Coeliac Patients Are Undiagnosed at Routine Upper Endoscopy

Coeliac disease

Persistent mucosal damage and risk of fracture in celiac disease.

Glutenase ALV003 Attenuates Gluten-Induced Mucosal Injury in Patients With Celiac Disease

Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity

Follow-up of pediatric celiac disease: value of antibodies in predicting mucosal healing, a prospective cohort study

Early feeding and risk of celiac disease in a prospective birth cohort

A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome

Celiac disease: diagnosis and management.

Usefulness of Symptoms to Screen for Celiac Disease

