Non-coeliac gluten sensitivity: the path towards diagnosis
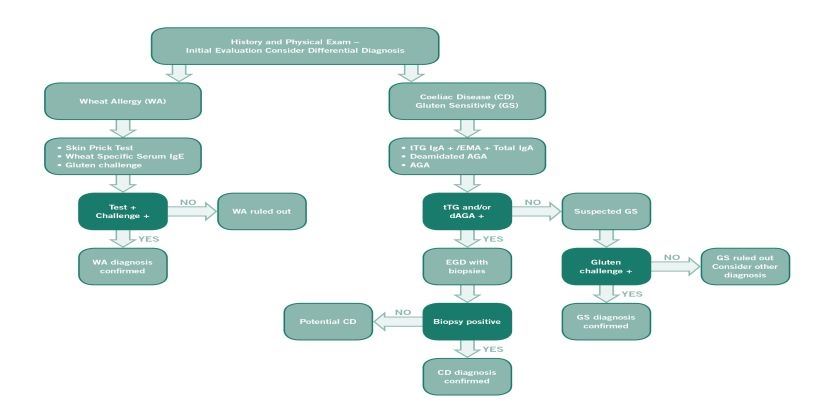
Diagnosis of non-coeliac gluten sensitivity (NCGS) requires assessment of improvement whilst following a gluten free diet, after coeliac disease and wheat allergy have been excluded.
If a patient reports non-specific symptoms, such as abdominal pain, bloating, diarrhoea, headaches, or fatigue after consuming foods containing gluten, it is important first to exclude coeliac disease and wheat allergy. The diagnosis is confirmed by an improvement or disappearance of symptoms with a gluten-free diet and a renewed deterioration once gluten is reintroduced. The symptoms usually improve within a few days to two weeks. It is important that the patient follows a diet that contains gluten before the diagnostic process begins.
Medical history
Identifying symptoms and signs
First, it is important to determine if the symptoms described by the patient are typical of NCGS and whether they could be related to the consumption of foods containing gluten. Before starting the diagnosis, the patient should follow a normal diet, i.e. one containing gluten, for at least six weeks.
First, it is important to determine if the symptoms described by the patient are typical of NCGS and whether they could be related to the consumption of foods containing gluten. Before starting the diagnosis, the patient should follow a normal diet, i.e. one containing gluten, for at least six weeks.
Excluding coeliac disease
Coeliac disease antibody test and biopsy
Since the symptoms of NCGS may resemble those of coeliac disease, it is important to exclude coeliac disease initially. It is crucial to ensure that patients are on a gluten containing diet at the time of investigation. The first step in excluding coeliac disease is coeliac serology (endomysial antibody or tissue transglutaminase antibody). A negative serological test has an excellent negative predictive value in excluding coeliac disease, provided the patient has been consuming a gluten containing diet for 6 weeks prior to the test. In cases with positive serology, a small intestine biopsy is required to confirm a diagnosis of coeliac disease [1]. Patients with NCGS will not have evidence of villous atrophy on duodenal biopsy, although the levels of intra-epithelial lymphocytes (IELs) may be slightly elevated (equating to Marsh scale 1) [2].
Gluten challenge to exclude coeliac disease
If patients are on a gluten free diet at the time of investigation consideration needs to be given to gluten challenge. Current guidelines recommend a 6 week challenge of 10 grams of gluten per day. However many patients with NCGS may not be able to tolerate this amount of gluten and recent evidence suggests that shorter challenges of 2 weeks with 3 grams of gluten are able to confirm coeliac disease in 75% of cases [3]. However duodenal biopsy is required in these cases.
Since the symptoms of NCGS may resemble those of coeliac disease, it is important to exclude coeliac disease initially. It is crucial to ensure that patients are on a gluten containing diet at the time of investigation. The first step in excluding coeliac disease is coeliac serology (endomysial antibody or tissue transglutaminase antibody). A negative serological test has an excellent negative predictive value in excluding coeliac disease, provided the patient has been consuming a gluten containing diet for 6 weeks prior to the test. In cases with positive serology, a small intestine biopsy is required to confirm a diagnosis of coeliac disease [1]. Patients with NCGS will not have evidence of villous atrophy on duodenal biopsy, although the levels of intra-epithelial lymphocytes (IELs) may be slightly elevated (equating to Marsh scale 1) [2].
Gluten challenge to exclude coeliac disease
If patients are on a gluten free diet at the time of investigation consideration needs to be given to gluten challenge. Current guidelines recommend a 6 week challenge of 10 grams of gluten per day. However many patients with NCGS may not be able to tolerate this amount of gluten and recent evidence suggests that shorter challenges of 2 weeks with 3 grams of gluten are able to confirm coeliac disease in 75% of cases [3]. However duodenal biopsy is required in these cases.
Excluding wheat allergy
IgE-antibody test and skin prick test
The clinical picture of wheat allergy may also be similar to that of NCGS. An IgE antibody test and skin prick test may be used to determine whether a patient is allergic to wheat.
The clinical picture of wheat allergy may also be similar to that of NCGS. An IgE antibody test and skin prick test may be used to determine whether a patient is allergic to wheat.
Other evidence of NCGS
IgG anti-gliadin antibody test
IgG anti-gliadin antibodies (AGAs) may be seen more frequently in NCGS [4]. However, AGAs may also be present in people with coeliac disease and in a small proportion of the healthy population. Research is ongoing to clarify the diagnostic procedure for NCGS in the future. At the present time IgG AGA cannot be used for diagnosis.
IgG anti-gliadin antibodies (AGAs) may be seen more frequently in NCGS [4]. However, AGAs may also be present in people with coeliac disease and in a small proportion of the healthy population. Research is ongoing to clarify the diagnostic procedure for NCGS in the future. At the present time IgG AGA cannot be used for diagnosis.
Gluten-free diet
Improvement in symptoms
If coeliac disease and wheat allergy have been ruled out, the patient may begin a gluten-free diet. In patients with NCGS, symptoms tend to improve or disappear within 14 days. However, it is necessary to follow a gluten-free diet for at least six weeks in order to be able to establish a causal relationship between a gluten-free diet and symptoms. In order to achieve standardisation, the improvement of symptoms should be assessed according to a diagnostic protocol developed at the International Expert Meeting 2014 [5]. This stipulates that the patient identifies 1 - 3 common symptoms and rates their severity on a scale of 1 - 10 before starting a gluten-free diet. This assessment should be carried out two weeks before starting the gluten-free diet and every week thereafter. A good response to the gluten-free diet is indicated by at least 30% improvement in any of the 3 symptoms. At least one symptom should improve, as long as the others do not deteriorate. This improvement should be achieved for a minimum of 50% of the evaluation period, i.e. in at least three of the six weekly reviews.
If coeliac disease and wheat allergy have been ruled out, the patient may begin a gluten-free diet. In patients with NCGS, symptoms tend to improve or disappear within 14 days. However, it is necessary to follow a gluten-free diet for at least six weeks in order to be able to establish a causal relationship between a gluten-free diet and symptoms. In order to achieve standardisation, the improvement of symptoms should be assessed according to a diagnostic protocol developed at the International Expert Meeting 2014 [5]. This stipulates that the patient identifies 1 - 3 common symptoms and rates their severity on a scale of 1 - 10 before starting a gluten-free diet. This assessment should be carried out two weeks before starting the gluten-free diet and every week thereafter. A good response to the gluten-free diet is indicated by at least 30% improvement in any of the 3 symptoms. At least one symptom should improve, as long as the others do not deteriorate. This improvement should be achieved for a minimum of 50% of the evaluation period, i.e. in at least three of the six weekly reviews.
Re-exposure to gluten
Confirming the diagnosis
As with food allergies, a renewed provocation may be necessary for a definitive diagnosis of NCGS. Following a period of at least 4 weeks on strict gluten free diet, patients should re-introduce gluten. If the symptoms recur within two days patients should restart a gluten free diet followed by a double-blind (for studies) or single-blind (in practice), placebo-controlled oral provocation. The most suitable foods for this purpose are gluten-free bars (as a placebo) and bars containing gluten that do not differ in appearance, texture or flavour. The first one-week trial period is followed by a one-week strict gluten-free diet and a second one-week trial period. The standardised diagnostic protocol should be used for the weekly assessment of symptoms. If a 30% reduction in 1 of the 3 symptom domains is demonstrated in the gluten free period compared to gluten containing period, gluten sensitivity is present.
As with food allergies, a renewed provocation may be necessary for a definitive diagnosis of NCGS. Following a period of at least 4 weeks on strict gluten free diet, patients should re-introduce gluten. If the symptoms recur within two days patients should restart a gluten free diet followed by a double-blind (for studies) or single-blind (in practice), placebo-controlled oral provocation. The most suitable foods for this purpose are gluten-free bars (as a placebo) and bars containing gluten that do not differ in appearance, texture or flavour. The first one-week trial period is followed by a one-week strict gluten-free diet and a second one-week trial period. The standardised diagnostic protocol should be used for the weekly assessment of symptoms. If a 30% reduction in 1 of the 3 symptom domains is demonstrated in the gluten free period compared to gluten containing period, gluten sensitivity is present.
Diagnostic protocol for gluten/wheat sensitivity
The aforementioned standardised diagnostic protocol was developed by leading researchers in the field of gluten-related disorders at the 3rd International Expert Meeting on Non-Celiac Gluten Sensitivity in October 2014 (5). This stipulates a uniform two-stage dietary process for which a modified version of the gastrointestinal symptom rating scale is used. Patients use this scale to assess the effects of removing and reintroducing gluten on their symptoms. Both gastrointestinal and extraintestinal symptoms are rated on a scale of 1 to 10. This questionnaire is designed to standardise diagnosis.
References
- Mooney, P. D., Hadjivassiliou, M. & Sanders, D. S. Coeliac disease. BMJ 348, g1561 (2014).
- Sapone, A. et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 9, 23 (2011).
- Leffler DA, Schuppan D, Pallav K et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013 Jul;62(7):996-1004.
- Caio, G., Volta, U., Tovoli, F. & De Giorgio, R. Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol. 14, 26 (2014).
- Catassi C, Elli L et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts' Criteria. Nutrients. 2015 Jun 18;7(6):4966-77.
Further information on this topic
Presentations
6
Show all
Studies
3
Show all
Expert interviews
2
Show all

How the diagnosis of non celiac gluten sensitivity should be confirmed: the Salerno experts’ criteria (2015)
Carlo Catassi
Department of Pediatrics
Università Politecnica delle Marche, Ancona
16th International Coeliac Disease Symposium 2015 in Prague
Pre-Conference Workshop on Gluten Sensitivity "The Evolving Planet of Gluten Related Disorders"
Department of Pediatrics
Università Politecnica delle Marche, Ancona
16th International Coeliac Disease Symposium 2015 in Prague
Pre-Conference Workshop on Gluten Sensitivity "The Evolving Planet of Gluten Related Disorders"

Biomarkers for Non-Celiac Gluten Sensitivity: A Tough, but Intriguing Challenge for Researchers (2015)
Umberto Volta
University of Bologna, Italy
16th International Coeliac Disease Symposium 2015 in Prague
Pre-Conference Workshop on Gluten Sensitivity "The Evolving Planet of Gluten Related Disorders"
University of Bologna, Italy
16th International Coeliac Disease Symposium 2015 in Prague
Pre-Conference Workshop on Gluten Sensitivity "The Evolving Planet of Gluten Related Disorders"

Diagnostic study of HLA-DQ2 typing for gluten sensitivity in IBS patients (2015)
Christian Barmeyer, Michael Schumann, Tim Meyer, Britta Siegmund, Severin Daum, Jörg-Dieter Schulzke, Reiner Ullrich
Medizinische Klinik für Gastroenterologie, Infektiologie und Rheumatologie
Charité - Universitätsmedizin Berlin
Campus Benjamin Franklin
16th International Coeliac Disease Symposium 2015 in Prague
Pre-Conference Workshop on Gluten Sensitivity "The Evolving Planet of Gluten Related Disorders"
Medizinische Klinik für Gastroenterologie, Infektiologie und Rheumatologie
Charité - Universitätsmedizin Berlin
Campus Benjamin Franklin
16th International Coeliac Disease Symposium 2015 in Prague
Pre-Conference Workshop on Gluten Sensitivity "The Evolving Planet of Gluten Related Disorders"

Gluten Sensitivity Biomarkers and Epidemiology, results from NHANES 2009–2010 (2013)
Peter H.R. Green
Celiac Disease Center
Columbia University
New York, USA
15. International Celiac Disease Symposium in Chicago between the 22nd and 25th September 2013
Celiac Disease Center
Columbia University
New York, USA
15. International Celiac Disease Symposium in Chicago between the 22nd and 25th September 2013

Gluten Sensitivity: Biomarkers and Epidemiology. The Ongoing Field Experience (2013)
Umberto Volta
Coordinator of Italian Celiac Association Board
Department of Medical and Surgical Sciences
University of Bologna, Italy
15. International Celiac Disease Symposium in Chicago between the 22nd and 25th September 2013
Coordinator of Italian Celiac Association Board
Department of Medical and Surgical Sciences
University of Bologna, Italy
15. International Celiac Disease Symposium in Chicago between the 22nd and 25th September 2013

Management of Gluten Sensitivity: Nutritional Management (2013)
Pam Cureton, RD, LD
Dietitian
Center for Celiac Research & Treatment
Boston MA, USA
15. International Celiac Disease Symposium in Chicago between the 22nd and 25th September 2013
Dietitian
Center for Celiac Research & Treatment
Boston MA, USA
15. International Celiac Disease Symposium in Chicago between the 22nd and 25th September 2013

How the diagnosis of non celiac gluten sensitivity should be confirmed: the Salerno experts’ criteria (2015)
Carlo Catassi
Department of Pediatrics
Università Politecnica delle ...

Biomarkers for Non-Celiac Gluten Sensitivity: A Tough, but Intriguing Challenge for Researchers (2015)
Umberto Volta
University of Bologna, Italy
16th International Coel...

Diagnostic study of HLA-DQ2 typing for gluten sensitivity in IBS patients (2015)
Christian Barmeyer, Michael Schumann, Tim Meyer, Britta Siegmund, Seve...

Gluten Sensitivity Biomarkers and Epidemiology, results from NHANES 2009–2010 (2013)
Peter H.R. Green
Celiac Disease Center
Columbia University
New York...

Gluten Sensitivity: Biomarkers and Epidemiology. The Ongoing Field Experience (2013)
Umberto Volta
Coordinator of Italian Celiac Association Board
Depart...

Management of Gluten Sensitivity: Nutritional Management (2013)
Pam Cureton, RD, LD
Dietitian
Center for Celiac Research & Treatment...

Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria
Abstract
Non-Celiac Gluten Sensitivity (NCGS) is a syndrome characterized by intestinal and extra-intestinal symptoms related to the ingestion of gluten-containing food, in subjects that are not affected by either celiac disease or wheat allergy. Given the lack of a NCGS biomarker, there is the need for standardizing the procedure leading to the diagnosis confirmation. In this paper we report experts’ recommendations on how the diagnostic protocol should be performed for the confirmation of NCGS. A full diagnostic procedure should assess the clinical response to the gluten-free diet (GFD) and measure the effect of a gluten challenge after a period of treatment with the GFD. The clinical evaluation is performed using a self-administered instrument incorporating a modified version of the Gastrointestinal Symptom Rating Scale. The patient identifies one to three main symptoms that are quantitatively assessed using a Numerical Rating Scale with a score ranging from 1 to 10. The double-blind placebo-controlled gluten challenge (8 g/day) includes a one-week challenge followed by a one-week washout of strict GFD and by the crossover to the second one-week challenge. The vehicle should contain cooked, homogeneously distributed gluten. At least a variation of 30% of one to three main symptoms between the gluten and the placebo challenge should be detected to discriminate a positive from a negative result. The guidelines provided in this paper will help the clinician to reach a firm and positive diagnosis of NCGS and facilitate the comparisons of different studies, if adopted internationally.
Resource: Nutrients 2015, 7(6), 4966-4977; doi:10.3390/nu7064966
Carlo Catassi, Luca Elli, Bruno Bonaz, Gerd Bouma, Antonio Carroccio, Gemma Castillejo, Christophe Cellier, Fernanda Cristofori, Laura de Magistris, Jernej Dolinsek, Walburga Dieterich, Ruggiero Francavilla, Marios Hadjivassiliou, Wolfgang Holtmeier, Ute Körner, Dan A. Leffler,Knut E. A. Lundin, Giuseppe Mazzarella, Chris J. Mulder, Nicoletta Pellegrini, Kamran Rostami, David Sanders, Gry Irene Skodje, Detlef Schuppan, Reiner Ullrich, Umberto Volta, Marianne Williams, Victor F. Zevallos, Yurdagül Zopf and Alessio Fasano
Non-Celiac Gluten Sensitivity (NCGS) is a syndrome characterized by intestinal and extra-intestinal symptoms related to the ingestion of gluten-containing food, in subjects that are not affected by either celiac disease or wheat allergy. Given the lack of a NCGS biomarker, there is the need for standardizing the procedure leading to the diagnosis confirmation. In this paper we report experts’ recommendations on how the diagnostic protocol should be performed for the confirmation of NCGS. A full diagnostic procedure should assess the clinical response to the gluten-free diet (GFD) and measure the effect of a gluten challenge after a period of treatment with the GFD. The clinical evaluation is performed using a self-administered instrument incorporating a modified version of the Gastrointestinal Symptom Rating Scale. The patient identifies one to three main symptoms that are quantitatively assessed using a Numerical Rating Scale with a score ranging from 1 to 10. The double-blind placebo-controlled gluten challenge (8 g/day) includes a one-week challenge followed by a one-week washout of strict GFD and by the crossover to the second one-week challenge. The vehicle should contain cooked, homogeneously distributed gluten. At least a variation of 30% of one to three main symptoms between the gluten and the placebo challenge should be detected to discriminate a positive from a negative result. The guidelines provided in this paper will help the clinician to reach a firm and positive diagnosis of NCGS and facilitate the comparisons of different studies, if adopted internationally.
Resource: Nutrients 2015, 7(6), 4966-4977; doi:10.3390/nu7064966
Carlo Catassi, Luca Elli, Bruno Bonaz, Gerd Bouma, Antonio Carroccio, Gemma Castillejo, Christophe Cellier, Fernanda Cristofori, Laura de Magistris, Jernej Dolinsek, Walburga Dieterich, Ruggiero Francavilla, Marios Hadjivassiliou, Wolfgang Holtmeier, Ute Körner, Dan A. Leffler,Knut E. A. Lundin, Giuseppe Mazzarella, Chris J. Mulder, Nicoletta Pellegrini, Kamran Rostami, David Sanders, Gry Irene Skodje, Detlef Schuppan, Reiner Ullrich, Umberto Volta, Marianne Williams, Victor F. Zevallos, Yurdagül Zopf and Alessio Fasano

Celiac Disease or Non-Celiac Gluten Sensitivity? An Approach to Clinical Differential Diagnosis
Abstract
OBJECTIVES: Differentiating between celiac disease (CD) and non-celiac gluten sensitivity (NCGS) is important for appropriate management but is often challenging.
METHODS: We retrospectively reviewed records from 238 patients who presented for the evaluation of symptoms responsive to gluten restriction without prior diagnosis or exclusion of CD. Demographics, presenting symptoms, serologic, genetic, and histologic data, nutrient deficiencies, personal history of autoimmune diseases, and family history of CD were recorded. NCGS was defined as symptoms responsive to a gluten-free diet (GFD) in the setting of negative celiac serology and duodenal biopsies while on a gluten-containing diet or negative human leukocyte antigen (HLA) DQ2/DQ8 testing.
RESULTS: Of the 238 study subjects, 101 had CD, 125 had NCGS, 9 had non-celiac enteropathy, and 3 had indeterminate diagnosis. CD subjects presented with symptoms of malabsorption 67.3% of the time compared with 24.8% of the NCGS subjects (P<0.0001). In addition, CD subjects were significantly more likely to have a family history of CD (P=0.004), personal history of autoimmune diseases (P=0.002), or nutrient deficiencies (P<0.0001). The positive likelihood ratio for diagnosis of CD of a >2× upper limit of normal IgA trans-glutaminase antibody (tTG) or IgA/IgG deaminated gliadan peptide antibody (DGP) with clinical response to GFD was 130 (confidence interval (CI): 18.5–918.3). The positive likelihood ratio of the combination of gluten-responsive symptoms and negative IgA tTG or IgA/IgG DGP on a regular diet for NCGS was 9.6 (CI: 5.5–16.9). When individuals with negative IgA tTG or IgA/IgG DGP also lacked symptoms of malabsorption (weight loss, diarrhea, and nutrient deficiencies) and CD risk factors (personal history of autoimmune diseases and family history of CD), the positive likelihood ratio for NCGS increased to 80.9.
CONCLUSIONS: On the basis of our findings, we have developed a diagnostic algorithm to differentiate CD from NCGS. Subjects with negative celiac serologies (IgA tTG or IgA/IgG DGP) on a regular diet are unlikely to have CD. Those with negative serology who also lack clinical evidence of malabsorption and CD risk factors are highly likely to have NCGS and may not require further testing. Those with equivocal serology should undergo HLA typing to determine the need for biopsy.
Resource: The American Journal of Gastroenterology 109, 741-746 (May 2014)
Toufic A Kabbani, Rohini R Vanga, Daniel A Leffler, Javier Villafuerte-Galvez, Kumar Pallav, Joshua Hansen, Rupa Mukherjee, Melinda Dennis and Ciaran P Kelly
OBJECTIVES: Differentiating between celiac disease (CD) and non-celiac gluten sensitivity (NCGS) is important for appropriate management but is often challenging.
METHODS: We retrospectively reviewed records from 238 patients who presented for the evaluation of symptoms responsive to gluten restriction without prior diagnosis or exclusion of CD. Demographics, presenting symptoms, serologic, genetic, and histologic data, nutrient deficiencies, personal history of autoimmune diseases, and family history of CD were recorded. NCGS was defined as symptoms responsive to a gluten-free diet (GFD) in the setting of negative celiac serology and duodenal biopsies while on a gluten-containing diet or negative human leukocyte antigen (HLA) DQ2/DQ8 testing.
RESULTS: Of the 238 study subjects, 101 had CD, 125 had NCGS, 9 had non-celiac enteropathy, and 3 had indeterminate diagnosis. CD subjects presented with symptoms of malabsorption 67.3% of the time compared with 24.8% of the NCGS subjects (P<0.0001). In addition, CD subjects were significantly more likely to have a family history of CD (P=0.004), personal history of autoimmune diseases (P=0.002), or nutrient deficiencies (P<0.0001). The positive likelihood ratio for diagnosis of CD of a >2× upper limit of normal IgA trans-glutaminase antibody (tTG) or IgA/IgG deaminated gliadan peptide antibody (DGP) with clinical response to GFD was 130 (confidence interval (CI): 18.5–918.3). The positive likelihood ratio of the combination of gluten-responsive symptoms and negative IgA tTG or IgA/IgG DGP on a regular diet for NCGS was 9.6 (CI: 5.5–16.9). When individuals with negative IgA tTG or IgA/IgG DGP also lacked symptoms of malabsorption (weight loss, diarrhea, and nutrient deficiencies) and CD risk factors (personal history of autoimmune diseases and family history of CD), the positive likelihood ratio for NCGS increased to 80.9.
CONCLUSIONS: On the basis of our findings, we have developed a diagnostic algorithm to differentiate CD from NCGS. Subjects with negative celiac serologies (IgA tTG or IgA/IgG DGP) on a regular diet are unlikely to have CD. Those with negative serology who also lack clinical evidence of malabsorption and CD risk factors are highly likely to have NCGS and may not require further testing. Those with equivocal serology should undergo HLA typing to determine the need for biopsy.
Resource: The American Journal of Gastroenterology 109, 741-746 (May 2014)
Toufic A Kabbani, Rohini R Vanga, Daniel A Leffler, Javier Villafuerte-Galvez, Kumar Pallav, Joshua Hansen, Rupa Mukherjee, Melinda Dennis and Ciaran P Kelly

Characterization of Adults With a Self-Diagnosis of Nonceliac Gluten Sensitivity
Abstract
Background: Nonceliac gluten sensitivity (NCGS), occurring in patients without celiac disease yet whose gastrointestinal symptoms improve on a gluten-free diet (GFD), is largely a self-reported diagnosis and would appear to be very common. The aims of this study were to characterize patients who believe they have NCGS.
Materials and Methods: Advertising was directed toward adults who believed they had NCGS and were willing to participate in a clinical trial. Respondents were asked to complete a questionnaire about symptoms, diet, and celiac investigation.
Results: Of 248 respondents, 147 completed the survey. Mean age was 43.5 years, and 130 were women. Seventy-two percent did not meet the description of NCGS due to inadequate exclusion of celiac disease (62%), uncontrolled symptoms despite gluten restriction (24%), and not following a GFD (27%), alone or in combination. The GFD was self-initiated in 44% of respondents; in other respondents it was prescribed by alternative health professionals (21%), dietitians (19%), and general practitioners (16%). No celiac investigations had been performed in 15% of respondents. Of 75 respondents who had duodenal biopsies, 29% had no or inadequate gluten intake at the time of endoscopy. Inadequate celiac investigation was common if the GFD was initiated by self (69%), alternative health professionals (70%), general practitioners (46%), or dietitians (43%). In 40 respondents who fulfilled the criteria for NCGS, their knowledge of and adherence to the GFD were excellent, and 65% identified other food intolerances.
Conclusions: Just over 1 in 4 respondents self-reporting as NCGS fulfill criteria for its diagnosis. Initiation of a GFD without adequate exclusion of celiac disease is common. In 1 of 4 respondents, symptoms are poorly controlled despite gluten avoidance.
Resource: Nutr Clin Pract April 16, 2014
Jessica R. Biesiekierski, PhD, RN, Evan D. Newnham , MD, FRACP, Susan J. Shepherd, PhD, APD, Jane G. Muir, PhD, APD, Peter R. Gibson, MD, FRACP
Background: Nonceliac gluten sensitivity (NCGS), occurring in patients without celiac disease yet whose gastrointestinal symptoms improve on a gluten-free diet (GFD), is largely a self-reported diagnosis and would appear to be very common. The aims of this study were to characterize patients who believe they have NCGS.
Materials and Methods: Advertising was directed toward adults who believed they had NCGS and were willing to participate in a clinical trial. Respondents were asked to complete a questionnaire about symptoms, diet, and celiac investigation.
Results: Of 248 respondents, 147 completed the survey. Mean age was 43.5 years, and 130 were women. Seventy-two percent did not meet the description of NCGS due to inadequate exclusion of celiac disease (62%), uncontrolled symptoms despite gluten restriction (24%), and not following a GFD (27%), alone or in combination. The GFD was self-initiated in 44% of respondents; in other respondents it was prescribed by alternative health professionals (21%), dietitians (19%), and general practitioners (16%). No celiac investigations had been performed in 15% of respondents. Of 75 respondents who had duodenal biopsies, 29% had no or inadequate gluten intake at the time of endoscopy. Inadequate celiac investigation was common if the GFD was initiated by self (69%), alternative health professionals (70%), general practitioners (46%), or dietitians (43%). In 40 respondents who fulfilled the criteria for NCGS, their knowledge of and adherence to the GFD were excellent, and 65% identified other food intolerances.
Conclusions: Just over 1 in 4 respondents self-reporting as NCGS fulfill criteria for its diagnosis. Initiation of a GFD without adequate exclusion of celiac disease is common. In 1 of 4 respondents, symptoms are poorly controlled despite gluten avoidance.
Resource: Nutr Clin Pract April 16, 2014
Jessica R. Biesiekierski, PhD, RN, Evan D. Newnham , MD, FRACP, Susan J. Shepherd, PhD, APD, Jane G. Muir, PhD, APD, Peter R. Gibson, MD, FRACP

Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria
Abstract
Non-Celiac Gluten Sensitivity (NCGS) is a syndrome character...

Celiac Disease or Non-Celiac Gluten Sensitivity? An Approach to Clinical Differential Diagnosis
Abstract
OBJECTIVES: Differentiating between celiac disease (CD) an...

Characterization of Adults With a Self-Diagnosis of Nonceliac Gluten Sensitivity
Abstract
Background: Nonceliac gluten sensitivity (NCGS), occurring...
NCGS: are we moving from a diagnosis of exclusion to a positive diagnosis?
Professor Carlo Catassi
Gastroenterologist Pediatrician
Department of Pediatrics
Università Politecnica delle Marche
60123 Ancona, Italy
At the Expert Meeting 2014 in Salerno, Italy.
Gastroenterologist Pediatrician
Department of Pediatrics
Università Politecnica delle Marche
60123 Ancona, Italy
At the Expert Meeting 2014 in Salerno, Italy.
Are we moving from a diagnosis of exclusion to a positive diagnosis?
Reiner Ullrich, MD
Universitätsmedizin Berlin
Campus Benjamin Franklin,
Medizinische Klinik für Gastroenterologie
12203 Berlin, Germany
At the Expert Meeting 2014 in Salerno, Italy
Universitätsmedizin Berlin
Campus Benjamin Franklin,
Medizinische Klinik für Gastroenterologie
12203 Berlin, Germany
At the Expert Meeting 2014 in Salerno, Italy

NCGS: are we moving from a diagnosis of exclusion to a positive diagnosis?
Professor Carlo Catassi
Gastroenterologist Pediatrician
Department o...

Are we moving from a diagnosis of exclusion to a positive diagnosis?
Reiner Ullrich, MD
Universitätsmedizin Berlin
Campus Benjamin Frankl...
www.drschaer-institute.com
