Therapie - Ernährungstherapie bei Zöliakie
Eine streng glutenfreie Ernährung ist aktuell die einzig mögliche Therapie bei Zöliakie.
Die einzige Lösung bzw. Therapie bei Zöliakie besteht darin, ein Leben lang auf alle glutenhaltigen Lebensmittel zu verzichten. Auch geringste Glutenmengen können Schäden an der Dünndarmschleimhaut verursachen, obwohl in manchen Fällen keine Symptome auftreten. Spuren von Gluten können in den verschiedensten verarbeiteten Lebensmitteln, beispielsweise Wurst, Gewürzzubereitungen oder Süßigkeiten, enthalten sein. Beim Einkauf und beim Außer-Haus-Verzehr ist deshalb besondere Vorsicht geboten.
Glutenfreie Produkte
Erleichtert wird die Umstellung auf eine glutenfreie Ernährung durch das breite Angebot an speziellen Produkten ohne Gluten, die für Zöliakiebetroffene geeignet sind. Im Lebensmittelhandel sind u. a. Brote, Nudeln und Mehlmischungen, aber auch Kekse und Knabbergebäck erhältlich, die mit dem Symbol der „durchgestrichenen Ähre“ gekennzeichnet sind. Dieses Symbol garantiert, dass ein Produkt glutenfrei ist.Ansprechen auf die glutenfreie Ernährung
Wird nach der Diagnosestellung mit einer streng glutenfreien Ernährung begonnen, werden bereits nach wenigen Wochen die ersten positiven Auswirkungen der Ernährungsumstellung sichtbar. Die Beschwerden verschwinden, das allgemeine Wohlbefinden und der gesundheitliche Zustand bessern sich und Gewichtsverluste werden ausgeglichen. Zusätzlich normalisieren sich die bei den serologischen Untersuchungen festgestellten Werte, die Dünndarmschleimhaut regeneriert und Mangelerscheinungen können ausgeglichen werden. Wer sich streng glutenfrei ernährt, kann beschwerdefrei leben. Außerdem mindert eine strikte glutenfreie Ernährung das Risiko langfristiger Gesundheitsprobleme, sowie das Auftreten von negativen Begleiterscheinungen und Komplikationen der Zöliakie.Vorteile der glutenfreien Ernährung
- Antikörperwerte normalisieren sich
- Dünndarmschleimhaut regeneriert sich
- Risiko für Folgeschäden sinkt
- Nährstoffe werden vom Körper wieder aufgenommen und verwertet
- Gewichtsverlust wird ausgegelichen
- Gesundheitlicher und körperlicher Zustand bessern sich
- Verbesserte Kontrolle, was gegessen wird
- Verbesserung der Lebensqualität, Wohlbefinden stellt sich wieder ein
- Kinder: normales Wachstum und eine gesunde Entwicklung
Vermeidung von Folgeerkrankungen
Wird Zöliakie nicht behandelt, kann es zu Folgeerscheinungen kommen. Durch Diätfehler und Nichteinhaltung einer strikt glutenfreien Ernährung erhöht sich das Risiko erheblich, an Spätfolgen der Zöliakie zu erkranken.
Mögliche Spätfolgen und Komplikationen der Zöliakie
- Osteoporose
- Malignome (Lymphome, Adenokarzinome)
- Kollagensprue
- Ulzerationen (Geschwüre im Darm)
Weiterführende Informationen
Download
5
Alle anzeigen
E-Learning
1
Alle anzeigen
Fachartikel
3
Alle anzeigen
Präsentationen
2
Alle anzeigen
Studien
3
Alle anzeigen

3-Stufen-Beratung III: Hinweise für den Berater
Ergänzend zu den Unterlagen, die durch das Beratungsgespräch führen, können sie hier kleine Notizzettel im DIN-A6-Format, mit weiterführenden Informationen und didaktischen Hinweisen herunterladen.

3-Stufen-Beratung I: Schulungsunterlagen Zöliakie
Hier können Sie alle Schulungsunterlagen zum Thema Zöliakie und glutenfreie Ernährung herunterladen. Laden Sie zusätzlich den "Hinweis für Berater" mit weiterführenden Informationen und didaktischen Hinweisen herunter.
Produktkatalog
Die Produktliste ist ausgerichtet für Fachkräfte. Hier sind die aktuell erhältlichen Produkte aufgelistet mit detaillierten Nährwerten und Zutatenangaben.

3-Stufen-Beratung III: Hinweise für den Berater
Ergänzend zu den Unterlagen, die durch das Beratungsgespräch führen, k...

3-Stufen-Beratung I: Schulungsunterlagen Zöliakie
Hier können Sie alle Schulungsunterlagen zum Thema Zöliakie und gluten...
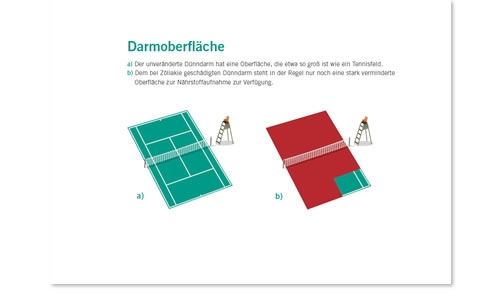
Infografik Tennisplatz

Kopiervorlage glutenfreier Speiseplan

Produktkatalog
Die Produktliste ist ausgerichtet für Fachkräfte. Hier sind die aktuel...

Modul 3 - Die glutenfreie Ernährung
In diesem Modul geht es um die glutenfreie Ernährung bei einer Zöliakie. Hier werden die konkrete Umsetzung einer glutenfreien Diät erläutert, das Modul führt in die 3-Stufen-Beratung ein und gibt wertvolle Tipps für die Praxis.
Die Lerninhalte wurden gemeinsam mit der Diplom Ökotrophologin Ute Körner erstellt.
Die Lerninhalte wurden gemeinsam mit der Diplom Ökotrophologin Ute Körner erstellt.

Modul 3 - Die glutenfreie Ernährung
In diesem Modul geht es um die glutenfreie Ernährung bei einer Zöliaki...

Das Darmmikrobiom im gesunden und kranken Zustand
Dank neuer Befunde und verbesserter analytischer Verfahren stehen immer mehr Informationen zu unserem Darmmikrobiom zur Verfügung. Es wird deutlich, dass die Art und relative Anzahl der Bakterien, die in unserem Darm vorkommen, eine Schlüsselrolle bei Gesundheit und Krankheit spielen.
>> Weiterlesen... <<<
>> Weiterlesen... <<<

Einfluss des Mikrobioms auf glutenbedingte Erkrankungen
Prof. Dr. med. Yurdagül Zopf und PD Dr. rer. nat. Walburga Dieterich berichten in ihrem Artikel über einen möglichen Zusammenhang zwischen Zöliakie und dem Mikrobiom sowie ihre derzeit laufende Studie, in der sie die Veränderungen der intestinalen Mikroflora bei Patienten mit nachgewiesener Gluten-/Weizensensitivität untersuchen.
>> Weiterlesen... <<<
>> Weiterlesen... <<<

Die Bedeutung der Mikrobiota bei der Entstehung und Therapie der Zöliakie
Dieser Artikel befasst sich mit dem Zusammenhang zwischen Mikrobiota und Zöliakie sowie dem Einsatz von Probiotika in der Therapie.
>> Weiterlesen... <<<
>> Weiterlesen... <<<

Das Darmmikrobiom im gesunden und kranken Zustand
Dank neuer Befunde und verbesserter analytischer Verfahren stehen imme...

Einfluss des Mikrobioms auf glutenbedingte Erkrankungen
Prof. Dr. med. Yurdagül Zopf und PD Dr. rer. nat. Walburga Dieterich b...

Die Bedeutung der Mikrobiota bei der Entstehung und Therapie der Zöliakie
Dieser Artikel befasst sich mit dem Zusammenhang zwischen Mikrobiota u...

Gluten and functional gastrointestinal disorders: is it worth the challenge? (2015)
Luca Elli MD, PhD
Center for the Prevention and Diagnosis of Celiac Disease
Fondazione IRCCS Ca’ Granda
Ospedale Maggiore Policlinico, Milano, Italy
16th International Coeliac Disease Symposium 2015 in Prag
Pre-Conference Workshop on Gluten Sensitivity "The Evolving Planet of Gluten Related Disorders"
Center for the Prevention and Diagnosis of Celiac Disease
Fondazione IRCCS Ca’ Granda
Ospedale Maggiore Policlinico, Milano, Italy
16th International Coeliac Disease Symposium 2015 in Prag
Pre-Conference Workshop on Gluten Sensitivity "The Evolving Planet of Gluten Related Disorders"

Glutenunverträglichkeiten erkennen und richtig behandeln (2014)
Prof. Dr. med. Yurdagül Zopf
Universitätsklinikum Erlangen
Universitätsklinikum Erlangen

Gluten and functional gastrointestinal disorders: is it worth the challenge? (2015)
Luca Elli MD, PhD
Center for the Prevention and Diagnosis of Celiac D...

Glutenunverträglichkeiten erkennen und richtig behandeln (2014)
Prof. Dr. med. Yurdagül Zopf
Universitätsklinikum Erlangen...

Potential Celiac Children: 9-Year Follow-Up on a Gluten-Containing Diet
Abstract
OBJECTIVES:
Potential celiac disease (CD) is defined by the presence of serum anti-tissue-transglutaminase (anti-TG2) antibodies and normal duodenal mucosa. The major clinical problem is the management of asymptomatic patients and how to predict the development of villous atrophy. This prospective longitudinal cohort study describes the natural history of potential CD up to 9 years and explores risk factors associated with the development of mucosal damage.
METHODS:
Two hundred and ten potential CD children were eligible for the study; 175/210 asymptomatic children were left on a gluten-containing diet. Antibodies and clinical symptoms were checked every 6 months, and a small bowel biopsy was taken every 2 years to evaluate histological, immunohistochemical, and anti-TG2 deposits. Patients were genotyped for HLA and a set of non-HLA CD-associated genes.
RESULTS:
Forty-three percent of patients showed persistently elevated anti-TG2 level, 20% became negative during follow-up, and 37% showed a fluctuant anti-TG2 course with transiently negative values. At 3 years of follow-up, 86% of cases remained potential; 73 and 67% still had normal duodenal architecture at 6 and 9 years, respectively. Male sex, slight mucosal inflammation at time 0, and a peculiar genetic profile delineate a cohort of individuals who were prone to develop mucosal damage during time.
CONCLUSIONS:
A sizeable proportion of asymptomatic potential celiac patients showed fluctuation or negativization of antibody production, and many of these, with persistently positive anti-TG2, did not develop mucosal damage after 9 years of follow-up. Celiac population is a multivariate aggregate of individuals with different genetic and phenotypic profiles. Caution is required before prescribing a gluten-free diet for life to asymptomatic individuals with potential CD.
Resource: The American Journal of Gastroenterology 109, 913-921 (June 2014)
Renata Auricchio, Antonella Tosco, Emanuela Piccolo, Martina Galatola, Valentina Izzo, Mariantonia Maglio, Francesco Paparo, Riccardo Troncone and Luigi Greco
OBJECTIVES:
Potential celiac disease (CD) is defined by the presence of serum anti-tissue-transglutaminase (anti-TG2) antibodies and normal duodenal mucosa. The major clinical problem is the management of asymptomatic patients and how to predict the development of villous atrophy. This prospective longitudinal cohort study describes the natural history of potential CD up to 9 years and explores risk factors associated with the development of mucosal damage.
METHODS:
Two hundred and ten potential CD children were eligible for the study; 175/210 asymptomatic children were left on a gluten-containing diet. Antibodies and clinical symptoms were checked every 6 months, and a small bowel biopsy was taken every 2 years to evaluate histological, immunohistochemical, and anti-TG2 deposits. Patients were genotyped for HLA and a set of non-HLA CD-associated genes.
RESULTS:
Forty-three percent of patients showed persistently elevated anti-TG2 level, 20% became negative during follow-up, and 37% showed a fluctuant anti-TG2 course with transiently negative values. At 3 years of follow-up, 86% of cases remained potential; 73 and 67% still had normal duodenal architecture at 6 and 9 years, respectively. Male sex, slight mucosal inflammation at time 0, and a peculiar genetic profile delineate a cohort of individuals who were prone to develop mucosal damage during time.
CONCLUSIONS:
A sizeable proportion of asymptomatic potential celiac patients showed fluctuation or negativization of antibody production, and many of these, with persistently positive anti-TG2, did not develop mucosal damage after 9 years of follow-up. Celiac population is a multivariate aggregate of individuals with different genetic and phenotypic profiles. Caution is required before prescribing a gluten-free diet for life to asymptomatic individuals with potential CD.
Resource: The American Journal of Gastroenterology 109, 913-921 (June 2014)
Renata Auricchio, Antonella Tosco, Emanuela Piccolo, Martina Galatola, Valentina Izzo, Mariantonia Maglio, Francesco Paparo, Riccardo Troncone and Luigi Greco

Follow-up of pediatric celiac disease: value of antibodies in predicting mucosal healing, a prospective cohort study
Abstract
Background: In diagnosing celiac disease (CD), serological tests are highly valuable. However, their role in following up children with CD after prescription of a gluten-free diet is unclear. This study aimed to compare the performance of antibody tests in predicting small-intestinal mucosal status in diagnosis vs. follow-up of pediatric CD.
Methods: We conducted a prospective cohort study at a tertiary-care center. 148 children underwent esophohagogastroduodenoscopy with biopsies either for symptoms ± positive CD antibodies (group A; n = 95) or following up CD diagnosed ≥ 1 year before study enrollment (group B; n = 53). Using biopsy (Marsh ≥ 2) as the criterion standard, areas under ROC curves (AUCs) and likelihood-ratios were calculated to estimate the performance of antibody tests against tissue transglutaminase (TG2), deamidated gliadin peptide (DGP) and endomysium (EMA).
Results: AUCs were higher when tests were used for CD diagnosis vs. follow-up: 1 vs. 0.86 (P = 0.100) for TG2-IgA, 0.85 vs. 0.74 (P = 0.421) for TG2-IgG, 0.97 vs. 0.61 (P = 0.004) for DPG-IgA, and 0.99 vs. 0.88 (P = 0.053) for DPG-IgG, respectively. Empirical power was 85% for the DPG-IgA comparison, and on average 33% (range 13–43) for the non-significant comparisons. Among group B children, 88.7% showed mucosal healing (median 2.2 years after primary diagnosis). Only the negative likelihood-ratio of EMA was low enough (0.097) to effectively rule out persistent mucosal injury. However, out of 12 EMA-positive children with mucosal healing, 9 subsequently turned EMA-negative.
Conclusions: Among the CD antibodies examined, negative EMA most reliably predict mucosal healing. In general, however, antibody tests, especially DPG-IgA, are of limited value in predicting the mucosal status in the early years post-diagnosis but may be sufficient after a longer period of time.
Resource: BMC Gastroenterology 2014, 14:28
Background: In diagnosing celiac disease (CD), serological tests are highly valuable. However, their role in following up children with CD after prescription of a gluten-free diet is unclear. This study aimed to compare the performance of antibody tests in predicting small-intestinal mucosal status in diagnosis vs. follow-up of pediatric CD.
Methods: We conducted a prospective cohort study at a tertiary-care center. 148 children underwent esophohagogastroduodenoscopy with biopsies either for symptoms ± positive CD antibodies (group A; n = 95) or following up CD diagnosed ≥ 1 year before study enrollment (group B; n = 53). Using biopsy (Marsh ≥ 2) as the criterion standard, areas under ROC curves (AUCs) and likelihood-ratios were calculated to estimate the performance of antibody tests against tissue transglutaminase (TG2), deamidated gliadin peptide (DGP) and endomysium (EMA).
Results: AUCs were higher when tests were used for CD diagnosis vs. follow-up: 1 vs. 0.86 (P = 0.100) for TG2-IgA, 0.85 vs. 0.74 (P = 0.421) for TG2-IgG, 0.97 vs. 0.61 (P = 0.004) for DPG-IgA, and 0.99 vs. 0.88 (P = 0.053) for DPG-IgG, respectively. Empirical power was 85% for the DPG-IgA comparison, and on average 33% (range 13–43) for the non-significant comparisons. Among group B children, 88.7% showed mucosal healing (median 2.2 years after primary diagnosis). Only the negative likelihood-ratio of EMA was low enough (0.097) to effectively rule out persistent mucosal injury. However, out of 12 EMA-positive children with mucosal healing, 9 subsequently turned EMA-negative.
Conclusions: Among the CD antibodies examined, negative EMA most reliably predict mucosal healing. In general, however, antibody tests, especially DPG-IgA, are of limited value in predicting the mucosal status in the early years post-diagnosis but may be sufficient after a longer period of time.
Resource: BMC Gastroenterology 2014, 14:28

Celiac disease: diagnosis and management.
Abstract
Celiac disease is an autoimmune disorder of the gastrointestinal tract. It is triggered by exposure to dietary gluten in genetically susceptible individuals. Gluten is a storage protein in wheat, rye, and barley, which are staples in many American diets. Celiac disease is characterized by chronic inflammation of the small intestinal mucosa, which leads to atrophy of the small intestinal villi and subsequent malabsorption. The condition may develop at any age. Intestinal manifestations include diarrhea and weight loss. Common extraintestinal manifestations include iron deficiency anemia, decreased bone mineral density, and neuropathy. Most cases of celiac disease are diagnosed in persons with extraintestinal manifestations. The presence of dermatitis herpetiformis is pathognomonic for celiac disease. Diagnosis is supported by a positive tissue transglutaminase serologic test but, in general, should be confirmed by a small bowel biopsy showing the characteristic histology associated with celiac disease. The presence of human leukocyte antigen alleles DQ2, DQ8, or both is essential for the development of celiac disease, and can be a useful genetic test in select instances. Treatment of celiac disease is a gluten-free diet. Dietary education should focus on identifying hidden sources of gluten, planning balanced meals, reading labels, food shopping, dining out, and dining during travel. About 5% of patients with celiac disease are refractory to a gluten-free diet. These patients should be referred to a gastroenterologist for reconsideration of the diagnosis or for aggressive treatment of refractory celiac disease, which may involve corticosteroids and immunomodulators.
Resource: Am Fam Physician. 2014 Jan 15;89(2):99-105.
Celiac disease is an autoimmune disorder of the gastrointestinal tract. It is triggered by exposure to dietary gluten in genetically susceptible individuals. Gluten is a storage protein in wheat, rye, and barley, which are staples in many American diets. Celiac disease is characterized by chronic inflammation of the small intestinal mucosa, which leads to atrophy of the small intestinal villi and subsequent malabsorption. The condition may develop at any age. Intestinal manifestations include diarrhea and weight loss. Common extraintestinal manifestations include iron deficiency anemia, decreased bone mineral density, and neuropathy. Most cases of celiac disease are diagnosed in persons with extraintestinal manifestations. The presence of dermatitis herpetiformis is pathognomonic for celiac disease. Diagnosis is supported by a positive tissue transglutaminase serologic test but, in general, should be confirmed by a small bowel biopsy showing the characteristic histology associated with celiac disease. The presence of human leukocyte antigen alleles DQ2, DQ8, or both is essential for the development of celiac disease, and can be a useful genetic test in select instances. Treatment of celiac disease is a gluten-free diet. Dietary education should focus on identifying hidden sources of gluten, planning balanced meals, reading labels, food shopping, dining out, and dining during travel. About 5% of patients with celiac disease are refractory to a gluten-free diet. These patients should be referred to a gastroenterologist for reconsideration of the diagnosis or for aggressive treatment of refractory celiac disease, which may involve corticosteroids and immunomodulators.
Resource: Am Fam Physician. 2014 Jan 15;89(2):99-105.

Potential Celiac Children: 9-Year Follow-Up on a Gluten-Containing Diet
Abstract
OBJECTIVES:
Potential celiac disease (CD) is defined by t...

Follow-up of pediatric celiac disease: value of antibodies in predicting mucosal healing, a prospective cohort study
Abstract
Background: In diagnosing celiac disease (CD), serological...

Celiac disease: diagnosis and management.
Abstract
Celiac disease is an autoimmune disorder of the gastrointe...
www.drschaer-institute.com

